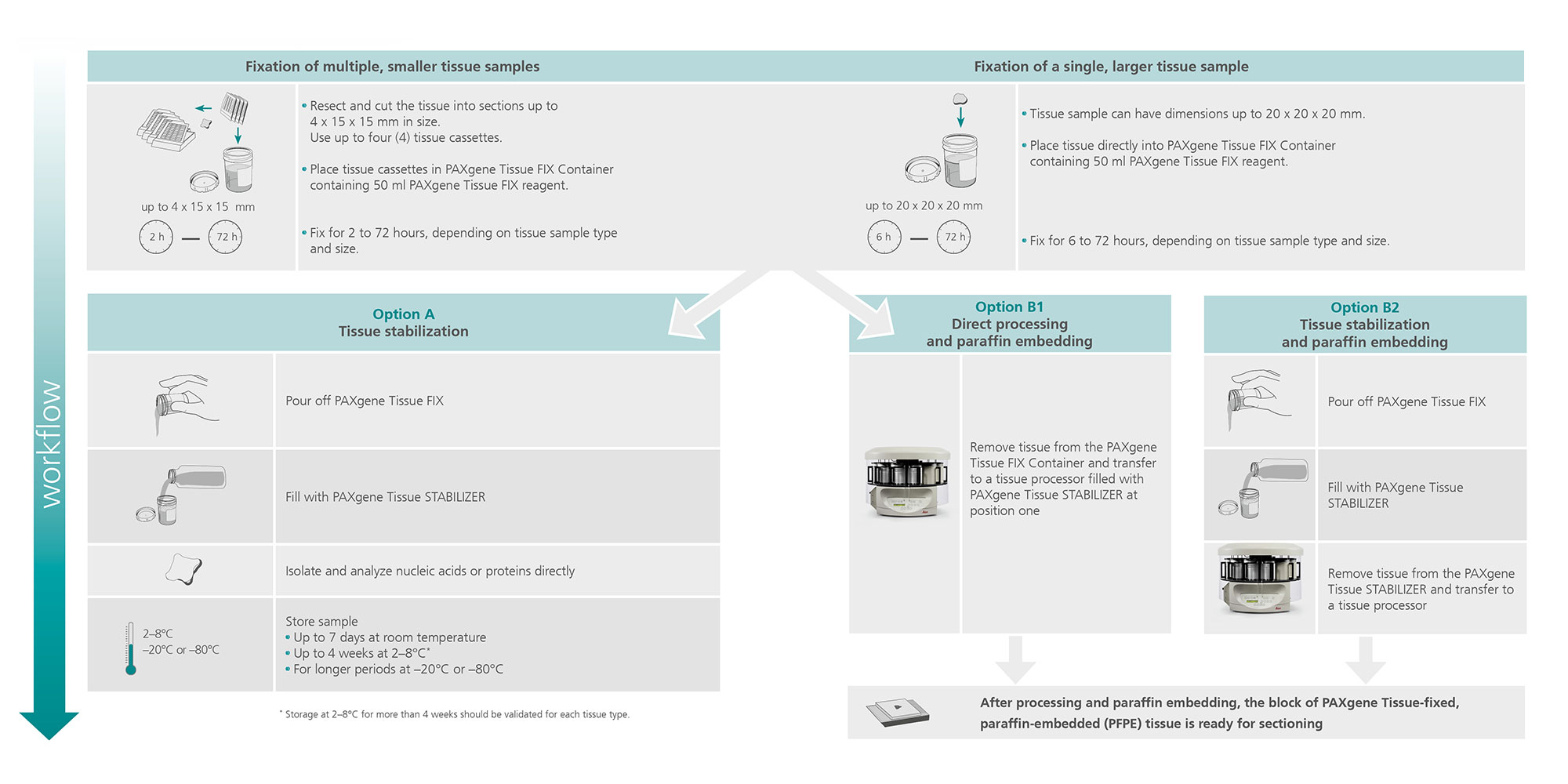
PAXgene Tissue FIX Container
Tissue fixation and stabilization chemistry
1. Which fixation method is used in the PAXgene Tissue System?
The PAXgene Tissue System uses an acidic and alcoholic fixation without formalin that does not result in crosslinking of biomolecules.
2. What is the composition of PAXgene Tissue FIX?
The PAXgene Tissue FIX fixation reagent contains alcohols and an acid, in addition to other stabilization agents.
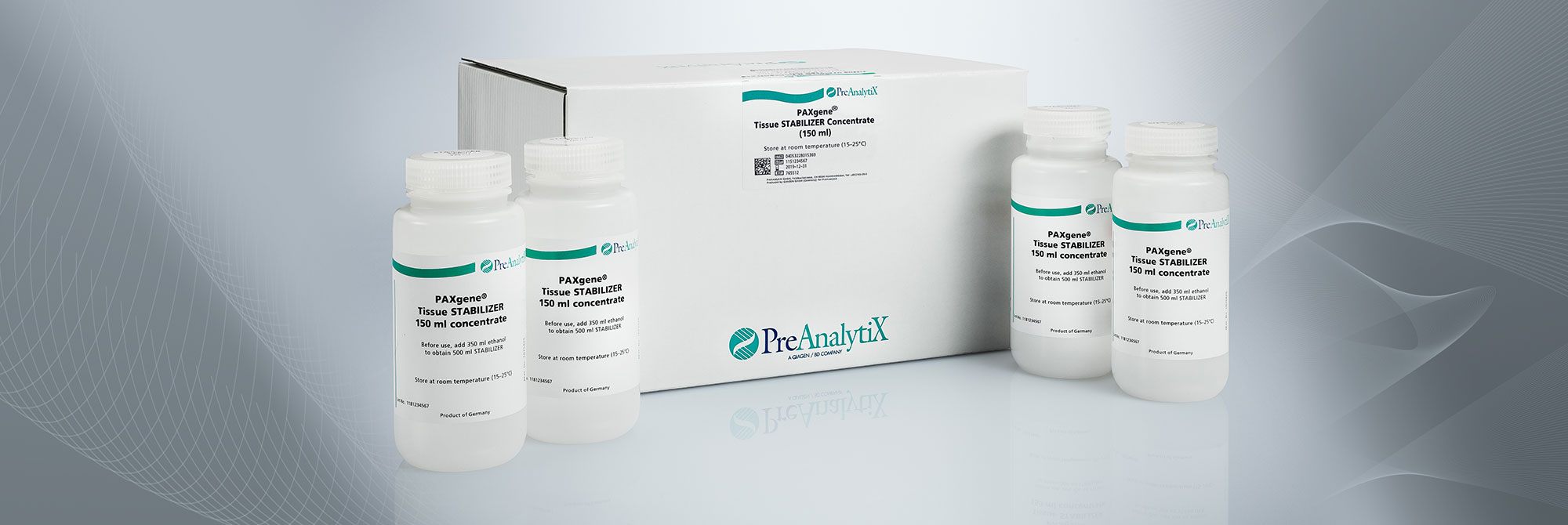
3. What is the composition of PAXgene Tissue STABILIZER?
The PAXgene Tissue STABILIZER stabilization reagent contains alcohol and other stabilization agents. It is available in bulk as a concentrate.
4. Are the two reagents used in the PAXgene Tissue System obligatory?
The PAXgene Tissue System involves two processes: fixation and stabilization. PAXgene Tissue FIX provides rapid penetration and fixation that effectively stops all enzymatic activity throughout the tissue. The tissue can remain in the fixative for maximum 72 h. For long-term transportation and storage, PAXgene Tissue STABILIZER stops fixation and stabilizes the specimen.
Tissue fixation and stabilization
1. What is the maximum tissue size that can be fixed in a PAXgene Tissue FIX Container (50 ml)?
Up to 4 standard tissue cassettes, each containing tissue samples with a maximum size of 4 x 15 x15 mm, or alternatively, a single tissue sample with a maximum size of 20 x 20 x 20 mm can be placed into a PAXgene Tissue FIX Container. If using a larger tissue sample surrounded by fat (e.g., from a lymph node) or a capsule (e.g., from kidney, liver or spleen), partially cut into the tissue every 5 mm (lamination) to enhance permeation of the fixation reagent. If samples are larger than recommended, the fast and even penetration of fixation reagent is compromised. This may result in quality reduction of tissue morphology and integrity of nucleic acids.
2. How long is the fixation time?
Depending on tissue size specimen(s) must be fixed at room temperature (15–25°C) for a minimum of 2 h (for samples up to 4 x 15 x 15 mm), or for a minimum of 6 h (for samples up to 20 x 20 x 20 mm). Fixation should be stopped by transfer into PAXgene Tissue STABILIZER after a maximum of 72 hours fixation.
For biopsies with a thickness of 1 mm or less, fixation time can be reduced to 30–60 min.
3. Which conditions are recommended for the storage of tissues in PAXgene Tissue STABILIZER?
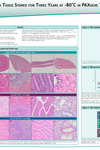
Depending on tissue type, standard storage conditions in PAXgene Tissue STABILIZER are up to 7 days at room temperature (15–25°C) or up to 4 weeks at 2–8°C. Storage at 2–8°C for more than 4 weeks must be validated for each tissue type. For longer storage, samples can be kept at–15°C to –30°C or –65°C to –90°C. Long-term storage studies are ongoing. For the latest results, see the poster "RNA and Morphology Preservation after 5 years at –20°C and 3 years at –80°C" under Resources.
4. Are PAXgene Tissue FIX Containers suitable for long-term storage at freezing temperature?
No. For storage at –15°C to –30°C or –65°C to –90°C use cryogenic vials with screw caps filled with PAXgene Tissue STABILIZER. For safety reasons, note that the PAXgene Tissue STABILIZER contains 70% ethanol.
Processing
1. Is it possible to use a standard processor – the kind used routinely for formalin-fixed samples – for dehydration and paraffin infiltration of PAXgene Tissue-treated samples?
Yes. All processors commonly used for formalin-fixed samples can be used to produce PAXgene Tissue-fixed, paraffin-embedded (PFPE) blocks of tissue.
We recommend keeping alcohol for processing PAXgene Tissue-treated samples separate from alcohol used for processing formalin-fixed samples for at least the first five positions/steps in the processing. With this precaution, it is possible to process PAXgene Tissue-fixed and formalin-fixed samples on the same instrument.
2. Is it possible to process formalin-fixed and PAXgene Tissue-fixed samples together in one run?
Parallel processing of formalin-fixed and PAXgene Tissue-fixed samples can lead to reduction of nucleic acid yield and integrity from PFPE samples through formalin contamination of reagents.
3. Is it necessary to clean a processor normally used for formalin-fixed tissue before using it with PAXgene Tissue-fixed tissue?
No. Special cleaning is not required. However, when processing PAXgene Tissue-treated specimens, do not use reagents contaminated with formalin. Residual formalin can lead to significant reduction of nucleic acid yield and integrity from PFPE tissue samples (see Technical Note "Influence of formalin contamination during processing of PAXgene Tissue fixed, paraffin-embedded tissue (PFPE) on RNA yield, integrity, and performance in quantitiative RT-PCR"). We recommend keeping alcohol for processing PAXgene Tissue-treated samples separate from alcohol used for processing formalin-fixed samples for at least the first five positions/steps in the processing. With this precaution, it is possible to process PAXgene Tissue-fixed and formalin-fixed samples on the same instrument.
4. Is a special processing protocol needed for the PAXgene Tissue System?
To prevent biomolecule degradation during processing, dehydration must start with at least 70–100% ethanol. We recommed using low-melting paraffin (melting point ≤56°C) and incubation in liquid paraffin for no longer than 3 hours.
Processing protocols for the PAXgene Tissue System are listed in the appendix of "PAXgene Tissue FIX Container (50 ml) Handbook".
5. Is it possible to integrate the PAXgene Tissue STABILIZER into automated tissue processing?
Yes. PAXgene Tissue STABILIZER can be used to fill the first position of a tissue processor. See the appendix of the "PAXgene Tissue FIX Container (50 ml) Handbook" for processing protocols with integrated PAXgene Tissue STABILIZER. When the STABILIZER is included as the first step of a protocol, tissues can be transferred from PAXgene Tissue FIX directly into the first processing position.
6. Is it possible to archive PAXgene Tissue-fixed, paraffin-embedded (PFPE) tissue blocks?
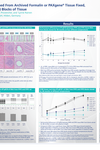
Tissue morphology is preserved in PFPE tissue when stored at room temperature. However, biomolecules within paraffin blocks will undergo slow chemical degradation. For best preservation of morphology and maintenance of biomolecule integrity within the paraffin-embedded tissue, store PFPE blocks refrigerated at 5°C (2–8°C) or ideally frozen at –20°C (–15°C to –30°C). See poster "RT-PCR Performance of RNA Obtained from Archived FFPE and PFPE Blocks of Tissue" under Resources.
7. Is it possible to embed samples fixed and stabilized with the PAXgene Tissue System in Optimal Cutting Temperature (OCT) medium for freezing?
Yes. PreAnalytiX has developed a workflow and protocols for cryo-embedding tissue specimens fixed and stabilized in the PAXgene Tissue FIX Container (50 ml). Supplementary protocols for generating PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues are available under Resources.
Compatibility with conventional pathology techniques
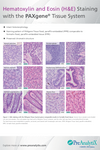
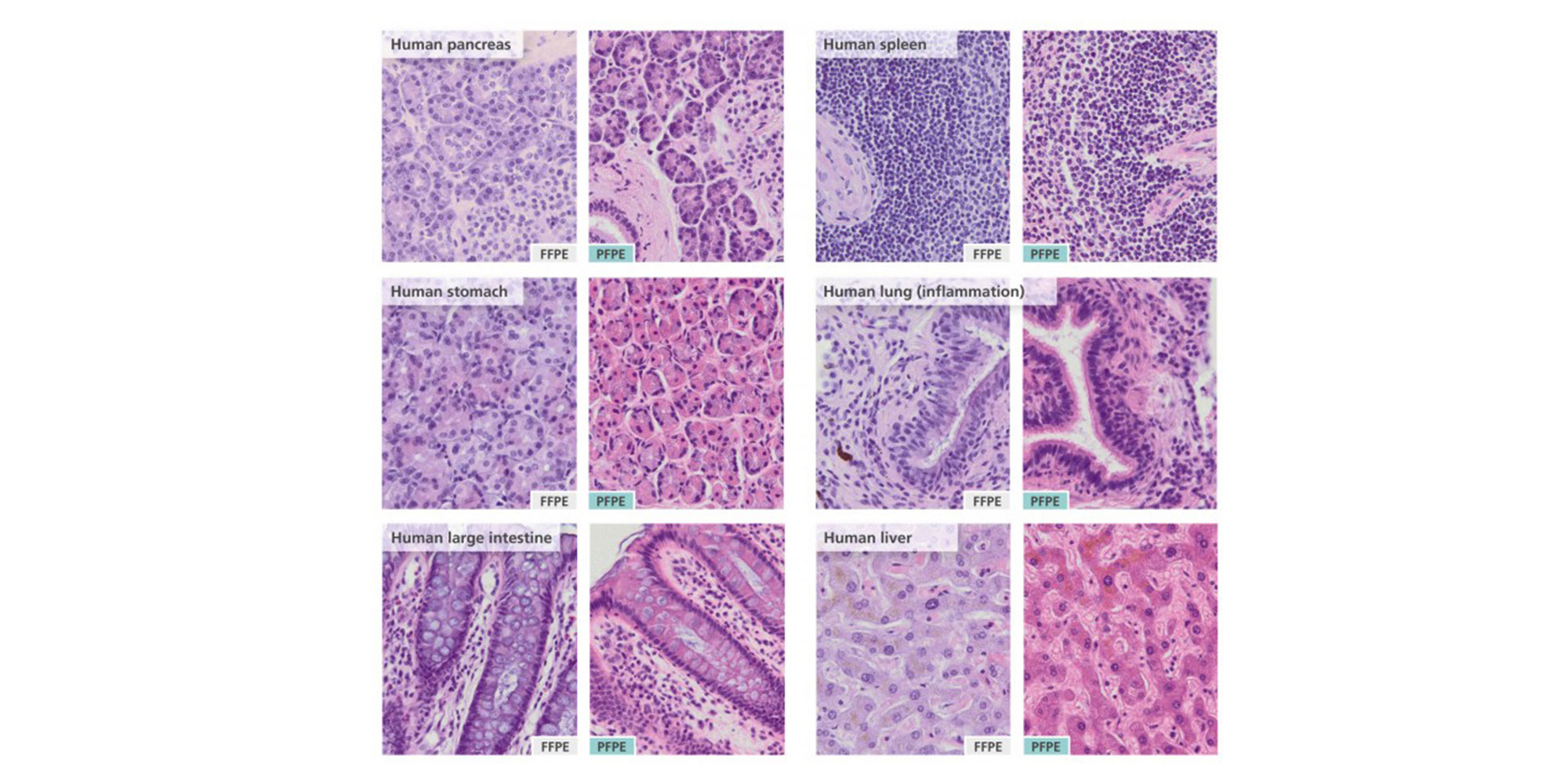
1. Is the morphology after H&E staining comparable to formalin-fixed samples?
Yes. Comparable morphology was observed in adjacent pieces from a tissue sample fixed either with neutral-buffered formalin or with the PAXgene Tissue System for a variety of human and animal tissues (Gündisch et al. 2014; Kap et al. 2011). Examples are provided in the Tissue Atlas. PAXgene Tissue treated-specimens have a tendency to be more eosinophilic. If an identical staining pattern to formalin-fixed samples is required, the incubation time in eosin should be reduced.
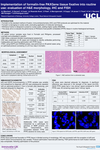
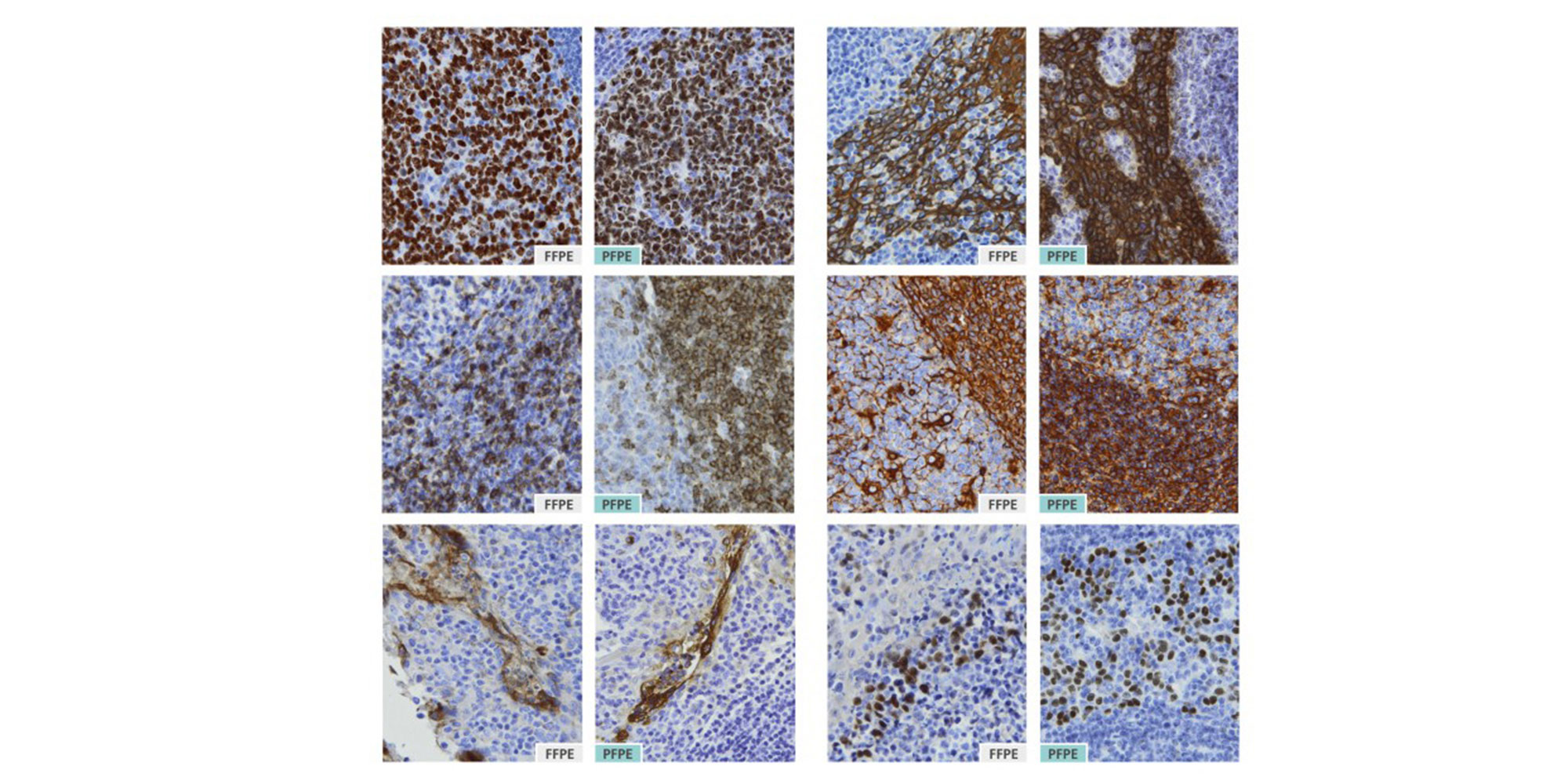
2. Are immunohistochemistry (IHC) assays developed for formalin-fixed, paraffin-embedded (FFPE) tissues compatible with PAXgene Tissue-fixed, paraffin-embedded (PFPE) tissues?
Most antibodies used in IHC assays were developed for use with formalin-fixed tissue and include steps for unmasking epitopes. When working with PAXgene Tissue-treated specimens, test each antibody to determine if it is necessary to perform antigen-retrieval steps. In addition, it may be necessary to optimize antigen-retrieval steps or adjust antibody concentrations in PFPE tissue to achieve optimal staining intensities (see Technical Note "Effect of epitope retrieval conditions on immunohistochemical staining of PFPE tonsil tissue with anti-human Ki-67 antigen (clone MIB-1)" under Resources). Examples for IHC staining of adjacent human tissue sections fixed with neutral-buffered formalin or with PAXgene Tissue reagents are provided in the Tissue Atlas.
3. Can sections of PAXgene Tissue fixed, paraffin-embedded (PFPE) tissue be used for other histochemical staining techniques, such as PAS?
Human tissue samples treated with the PAXgene Tissue System were successfully used for periodic acid schiff (PAS), resorcin fuchsin, sirius red and Gomori staining (Kap et al. 2011). However, to achieve the same staining intensities with both PFPE and FFPE samples, it may be necessary to adjust incubation times.
4. Can PAXgene Tissue-fixed, paraffin-embedded tissue be used for in situ hybridization?
Yes, human tissue samples treated with the PAXgene Tissue System have been successfully used for fluorescence in situ hybridization (FISH). See the supplementary protocol "Preparation of PFPE tissue sections for use with in situ hybridization (ISH) staining" and Oberauner-Wappis et al. 2016 under Resources.
Purification and quality of biomolecules from PAXgene Tissue-treated samples
1. Is it possible to use formalin-fixed, paraffin-embedded (FFPE) kits and protocols to isolate biomolecules from PAXgene Tissue-fixed, paraffin-embedded (PFPE) tissues?
No. Procedures developed for the extraction of biomolecules from FFPE tissues include prolonged proteinase K digestion and heating steps to remove chemical modifications introduced by formalin. Since the PAXgene Tissue System does not chemically modify biomolecules, these steps are not necessary and, in fact, lead to degradation of biomolecules. Instead, use dedicated PAXgene Tissue kits and supplementary protocols for extraction of biomolecules from PAXgene Tissue-treated samples.
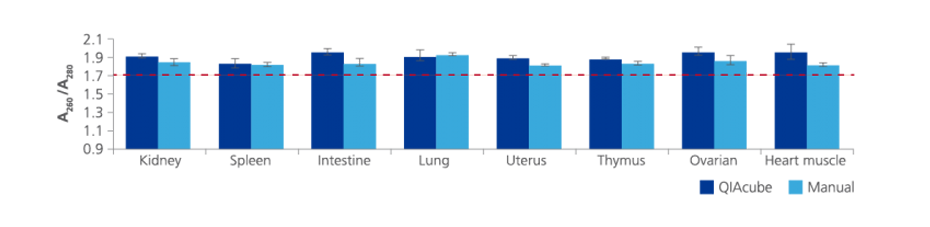
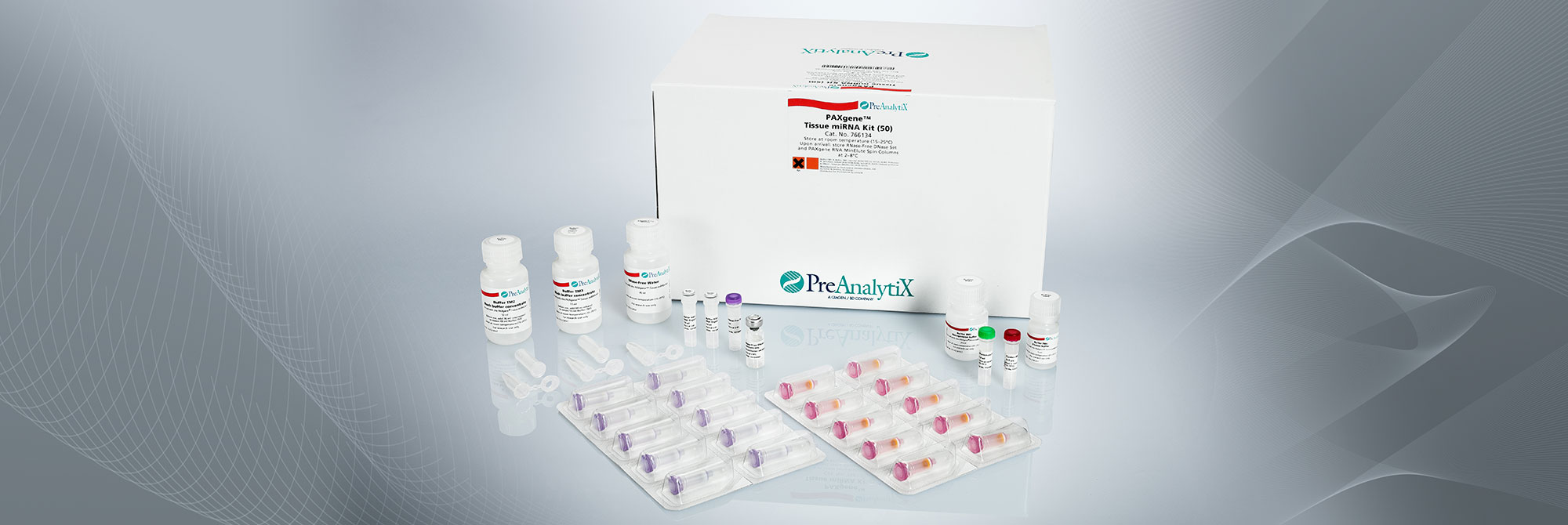
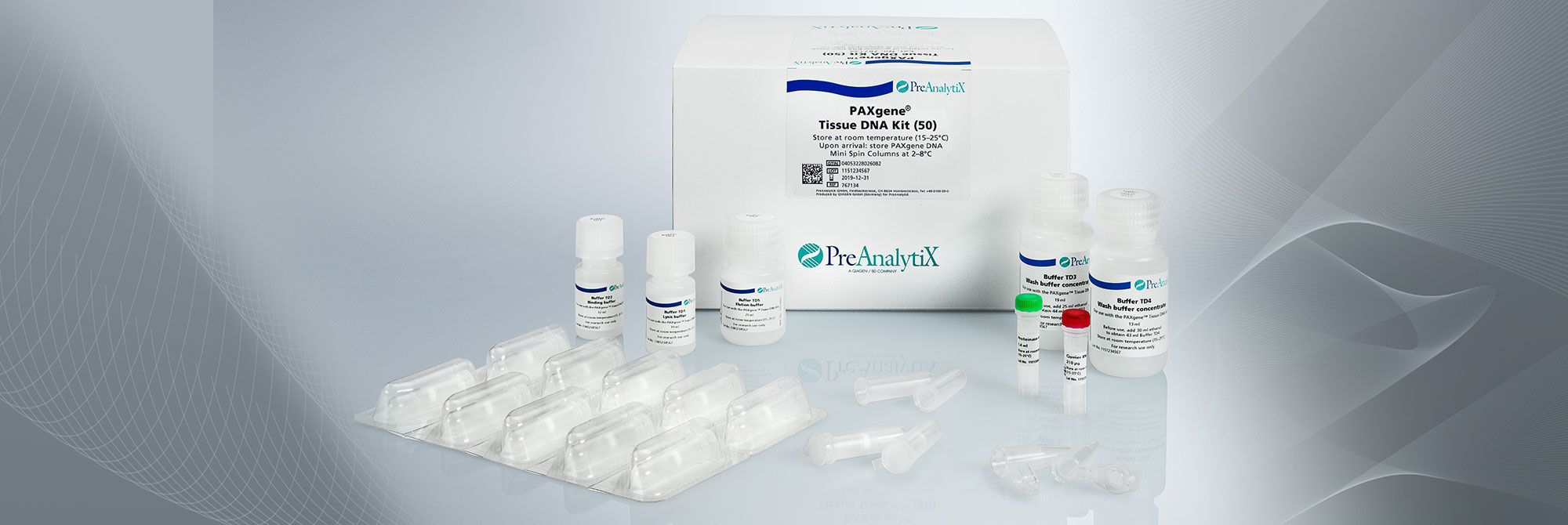
2. What is the purity of nucleic acids extracted with the PAXgene Tissue Kits?
The PAXgene Tissue DNA and RNA/miRNA Kits are based on proven QIAGEN technologies. Nucleic acids isolated with these kits are generally of high purity.
On average, measurements of the A260/A280 ratio for DNA purified with the PAXgene Tissue DNA Kit are >1.7, and ratios for RNA including miRNA purified with the PAXgene Tissue RNA/ miRNA Kit are >1.8.
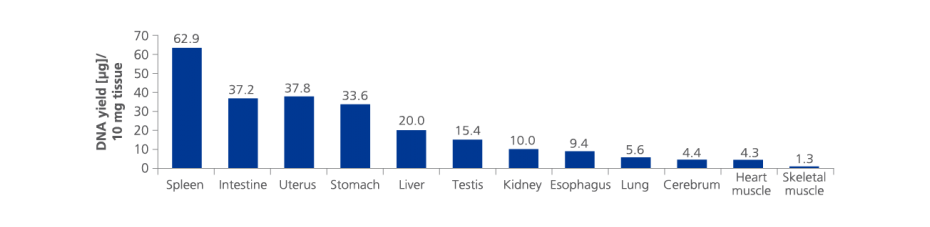
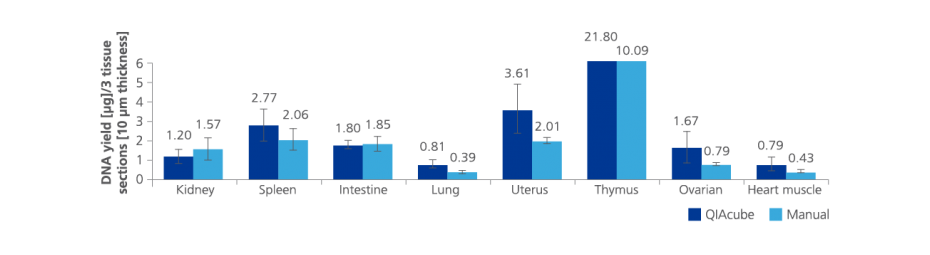
3. What is the average RNA yield from PAXgene Tissue fixed, paraffin-embedded (PFPE) tissues?
RNA yield, including miRNA, depends on several parameters, such as tissue type, time from resection until fixation, fixation time, processing protocol used and age and storage conditions of the PFPE block.
In a study with PFPE tissue sections (area: 100 mm²; thickness: 10 µm) median RNA yield from rat liver was 4.2 µg (n=58), from kidney 2.2 µg (n=58), from spleen 4.7 µg (n=58), from intestine 4.7 µg (n=58) and from lung 0.9 µg (n=58). See the Technical Note "Yield, purity, and integrity of RNA purified from PAXgene Tissue fixed, paraffin-embedded (PFPE) rat tissue" under Resources.
4. How well is RNA integrity preserved in PAXgene Tissue-fixed, paraffin-embedded (PFPE) tissues?
Similar to yield, RNA integrity depends on several parameters, such as tissue type, time from resection until fixation, fixation time, processing protocol and age and storage conditions of the PFPE block. For examples of RNA integrity values from rat tissues under ideal workflow conditions, see Groelz et al. 2013. For examples of RNA integrity from clinical samples, see Viertler et al. 2012.
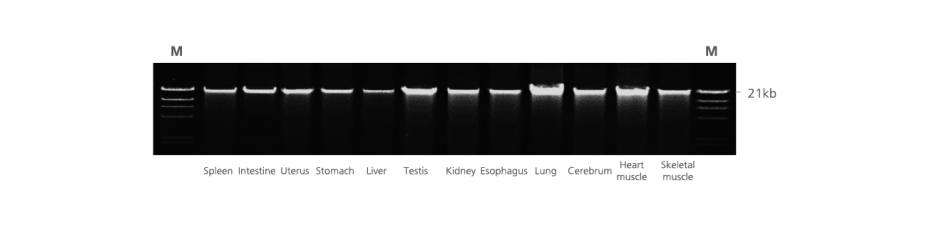
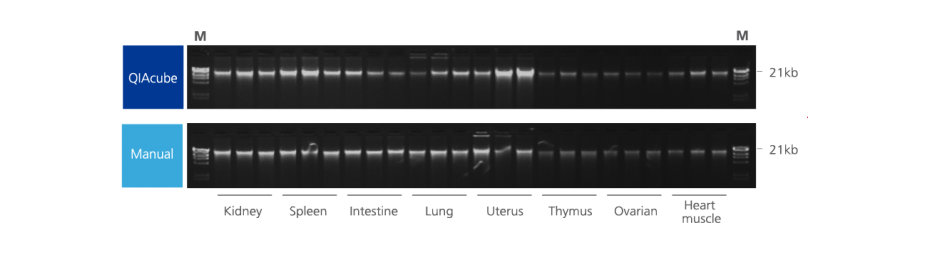
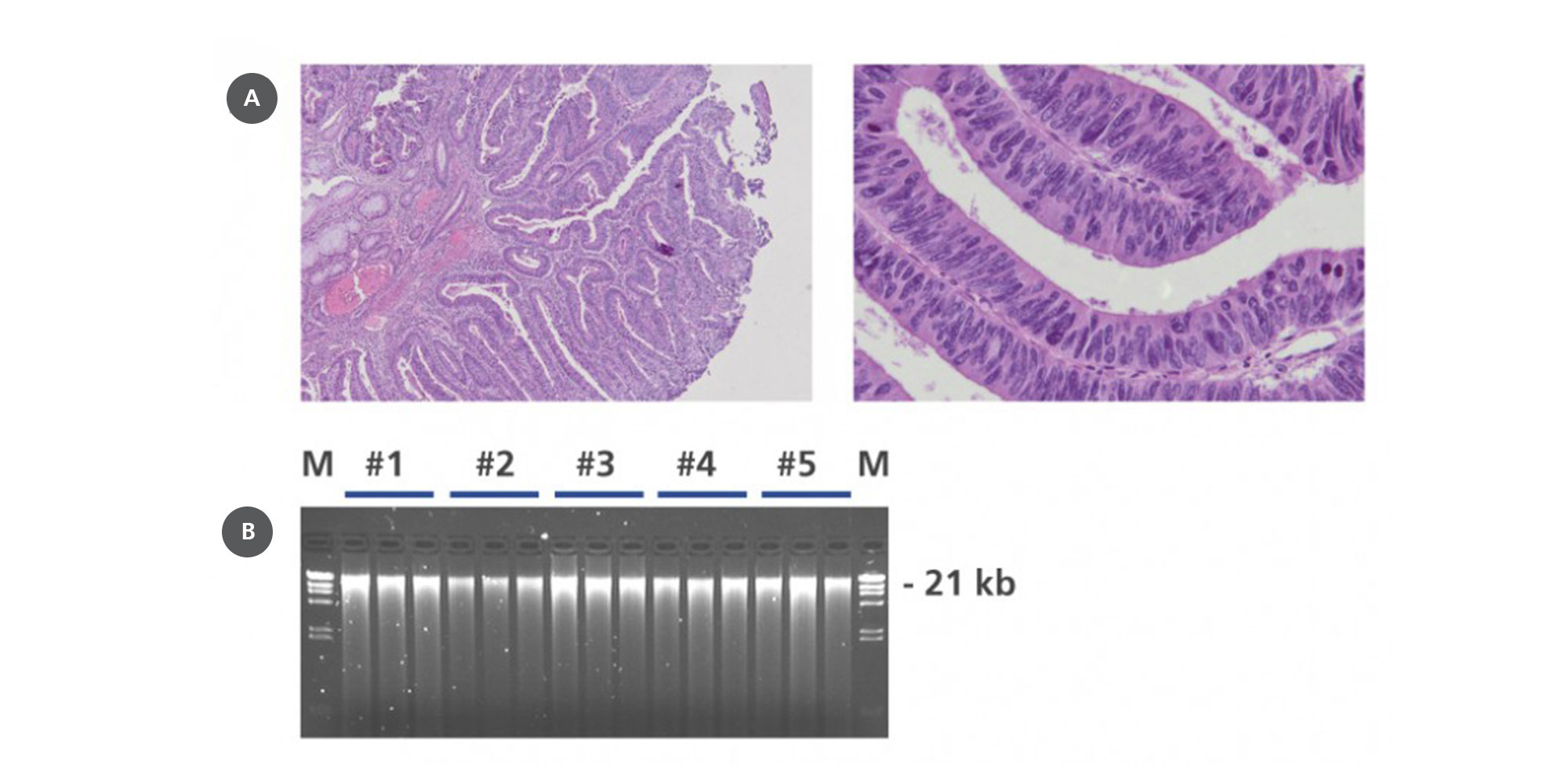
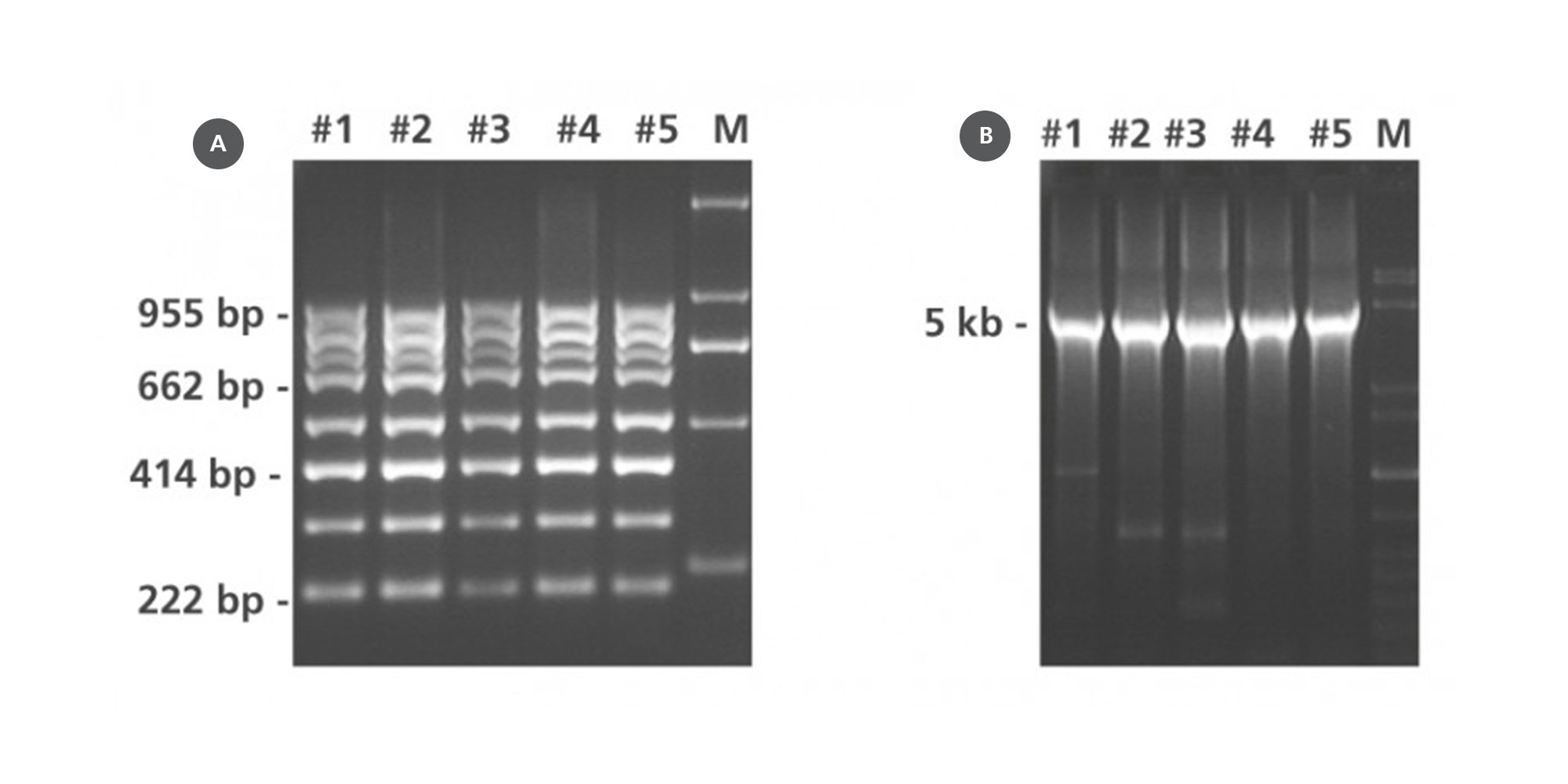
5. How well is DNA integrity preserved in PAXgene Tissue-fixed, paraffin-embedded (PFPE) tissues?
In contrast to DNA isolated from formalin-fixed, paraffin-embedded (FFPE) tissue, DNA from PFPE tissue exhibits high molecular weight. In most cases, a distinct 10 kb band is observed in electrophoretically separated DNA eluates. For an example, see Figure 2 in the Technical Note “Quantitative analysis of KRAS and BRAF mutational status in DNA from PAXgene Tissue fixed, paraffin-embedded (PFPE) tissue using Pyrosequencing technology“ under Resources.
6. What are the yield and integrity of nucleic acids isolated from blocks of PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues?
DNA and RNA isolated from PFCE tissue specimens is of high quantity and quality, comparable to PFPE tissue.
7. Are special kits and protocols required for the isolation of biomolecules from PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues?
No. Regular PAXgene Tissue Kits can be used for the isolation of RNA, including miRNA, and DNA from PFCE tissue. Supplementary protocols developed specifically for the extraction of biomolecules from PFCE samples are available under Resources.
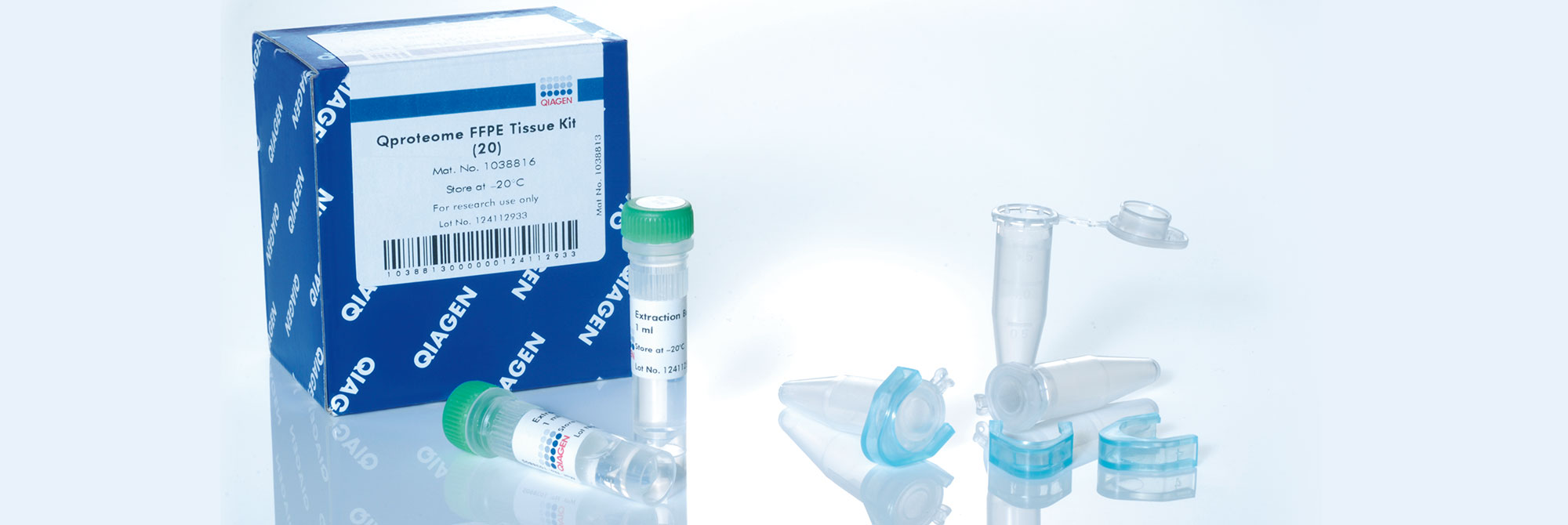
8. Can proteins be extracted from PAXgene Tissue-fixed specimens?
Yes. Supplementary protocols are available for the purification of full-length proteins from PAXgene Tissue fixed (PF) tissue and paraffin blocks using the Qproteome FFPE Tissue Kit (QIAGEN, cat. no. 37623). For more information, see the corresponding supplementary protocols under Resources.
9. Is it possible to microdissect PAXgene Tissue-fixed, paraffin-embedded (PFPE) and PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues?
Yes, supplementary protocols for generating sections from PFPE and PFCE tissue blocks for manual and laser microdissection are available under Resources.
10. Which kits and protocols can be used for the isolation of nucleic acids from microdissected PAXgene Tissue-fixed, paraffin-embedded (PFPE) and PAXgene Tissue-fixed, cryo-embedded (PFCE) specimens?
Regular PAXgene Tissue kits can be used for the isolation of total RNA, including miRNA, and DNA from microdissected PFPE and PFCE tissue. Supplementary protocols developed specifically for the extraction of biomolecules from microdissected PFPE and PFCE samples are available under Resources.
Molecular analysis of biomolecules purified from PAXgene Tissue-treated samples
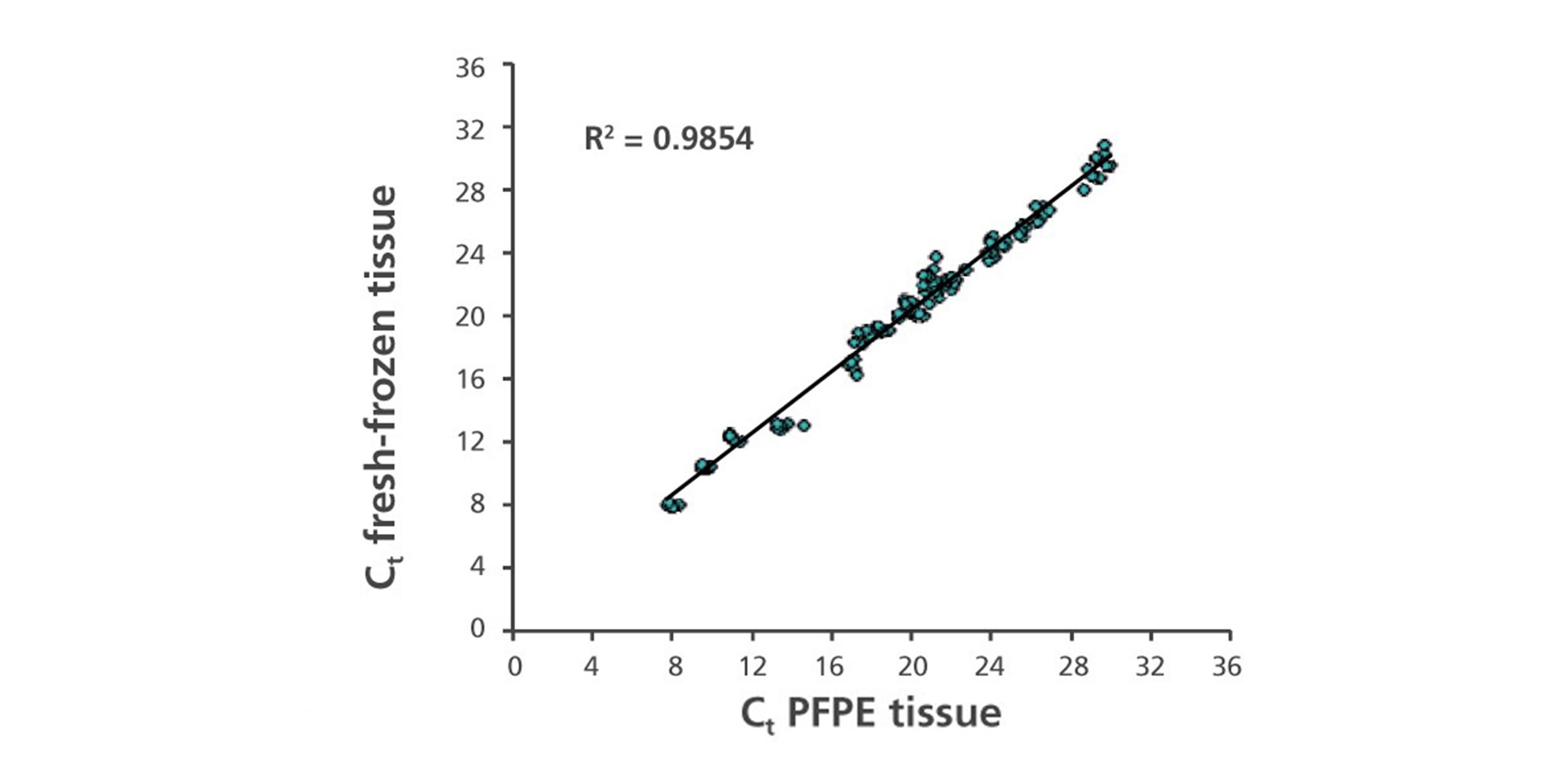
1. What is the RT-PCR performance of RNA purified from PAXgene Tissue-fixed, paraffin-embedded (PFPE) and PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues compared to RNA from snap-frozen or formalin-fixed, paraffin-embedded (FFPE) tissues?
RNA, including miRNA, purified from PFPE and PFCE is free of chemical modifications and performs similarly or identically to RNA isolated from frozen tissue. For examples of the correlation of gene expression levels in snap frozen tissue, FFPE, and PFPE, see Figure 4 in Groelz et al. 2013 and Figure 3 in Viertler et al. 2012.
2. What is the PCR performance of DNA purified from PAXgene Tissue-fixed, paraffin-embedded (PFPE) and PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues compared to DNA from snap-frozen or formalin-fixed paraffin-embedded (FFPE) tissues?
In contrast to FFPE, DNA purified from PFPE and PFCE is of high molecular weight and free of chemical modifications. In demanding downstream applications, such as multiplex or long-range PCR, it performs similarly or identically to DNA isolated from frozen tissue. For examples, see, Figure 5 in Viertler et al. 2012.
3. Is DNA purified from PAXgene Tissue-fixed, paraffin-embedded (PFPE) tissues suitable for targeted NGS analysis?
Yes. DNA purified from PFPE is of high molecular weight and free of chemical modifications. Several independent researchers successfully used DNA from PFPE for NGS with different sample types, workflows and platforms (data in preparation). The data confirm recent suggestions that some DNA sequence artifacts associated with FFPE can be avoided with PFPE (Högnäs et al. 2017).
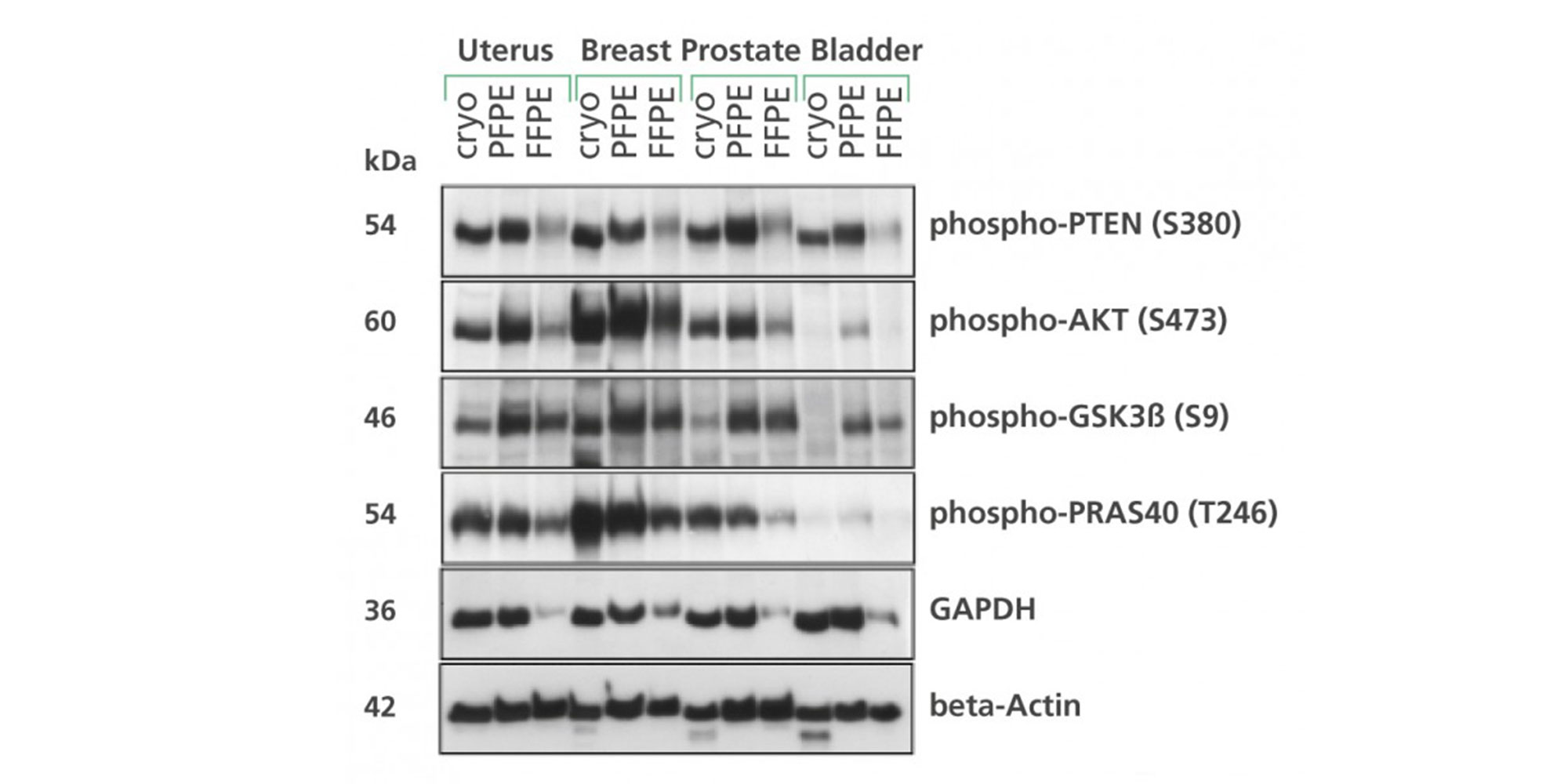
4. How is the quality of proteins purified from tissues fixed and stabilized with the PAXgene Tissue System?
Proteins from PAXgene Tissue-fixed, paraffin-embedded (PFPE) and PAXgene Tissue-fixed, cryo-embedded (PFCE) tissues are non-degraded, immunoreactive and have been successfully investigated by western blot analysis, reverse-phase protein arrays, two-dimensional gel electrophoresis (2D-PAGE), enzyme-linked immunosorbent assay (ELISA) and MALDI imaging mass spectrometry.
![Figure 7. RNA purified without chemical modification from PFPE tissue using the PAXgene Tissue RNA/miRNA Kit SYBR Green real-time RT-qPCR was performed with 10 ng RNA from cryopreserved, formalin-fixed, paraffin-embedded (FFPE) or PAXgene Tissue-fixed, paraffin-embedded (PFPE) rat tissue (modified according to Groelz et al. 2013). Depicted are the average delta-Ct values (delta-Ct = Ct[FFPE] – Ct[cryo] or delta-Ct = Ct[PFPE] – Ct[cryo]) from 6 different assays with amplicons ranging from 109 to 465 bp.](/storage/images/Content/Product_pages/Tissue/Fixation_Stabilization/SYBR_Green_real-tome_RT-qPCR_FFPE_PFPE.jpg)