PAXgene Bone Marrow RNA Kit
1. Can the PAXgene Bone Marrow RNA Kit be used for diagnostic or prognostic procedures?
No. The product is ‘For Research Use.’ Not for use in diagnostic procedures. No claim or representation is intended to provide information for the diagnosis, prevention, or treatment of a disease.
2. What RNA yields are expected with this product?
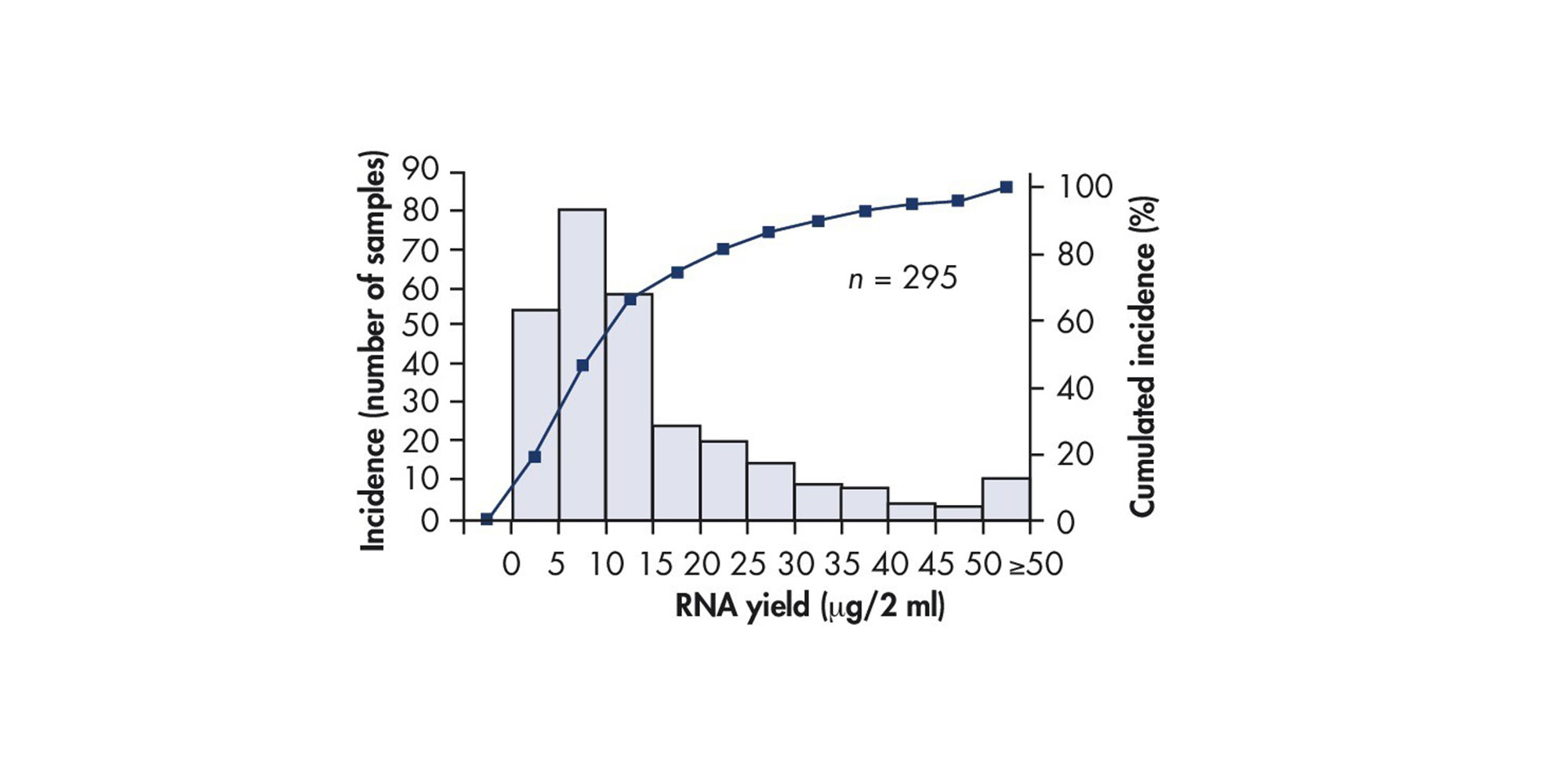
Bone marrow samples are extremely heterogeneous, consisting of varying amounts of cells, tissue and bone fragments. RNA yields are highly donor-dependent and can vary greatly from sample to sample, as well as between replicates from the same donor, due to sample-intrinsic heterogeneity. In a study of 295 bone marrow samples, yields of RNA isolated from 2 ml of human bone marrow were between 2.0 and 44.7 µg (5–95% quantiles, mean = 15.8 µg, median = 11.0 µg).
3. Can the PAXgene Bone Marrow RNA System be used to isolate viral RNA?
No. The PAXgene Bone Marrow RNA System has been optimized only for cellular RNA.
4. Can DNA be isolated with the kit as well?
No. The PAXgene Bone Marrow RNA System has been optimized only for cellular RNA.
5. Can specific types of white blood cells be enriched from a PAXgene Bone Marrow RNA Tube prior to the RNA isolation?
No. The cells are lysed in the PAXgene Bone Marrow RNA Tube. There is no method available to enrich certain subpopulations of cells after bone marrow is transferred to the PAXgene Bone Marrow RNA Tube.
6. Is RNA from red blood cells also isolated when RNA is extracted from a PAXgene Bone Marrow RNA Tube?
Yes. The PAXgene Bone Marrow RNA System purifies RNA from whole bone marrow. Therefore it also purifies RNA from other cells, e.g., reticulocytes and RBCs. Array analyses have shown higher globin mRNA levels for RNA isolated from whole bone marrow compared to RNA isolated from mononuclear cells.
7. Can bone marrow from leukemia patients with elevated numbers of white blood cells be processed?
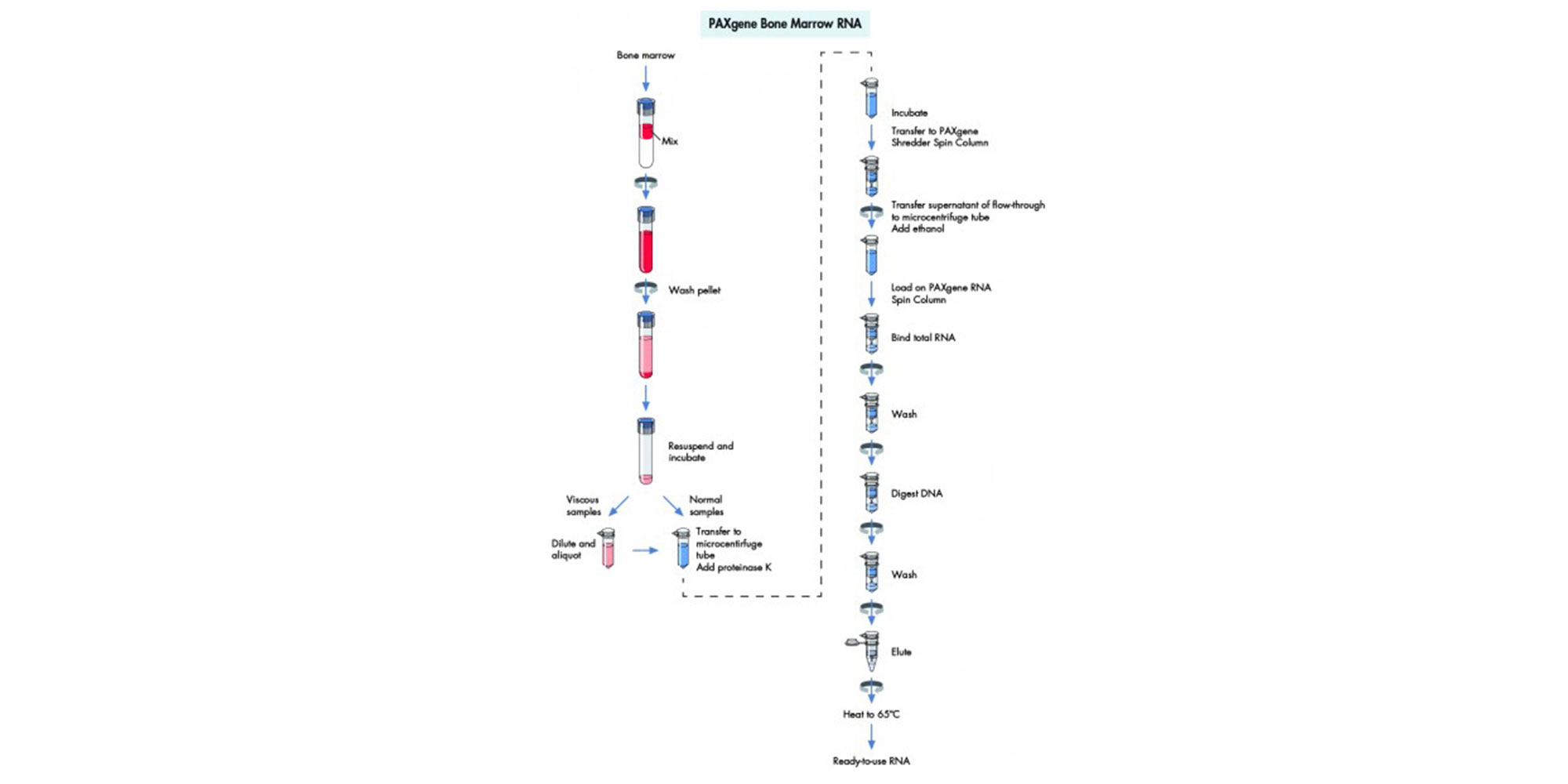
Yes. We have tested the system with bone marrow samples containing very high numbers of leukocytes. Bone marrow samples with higher leukocyte numbers are indicated by a highly viscous solution after addition of buffers BMR1 and BMR2 followed by incubation. To obtain high-quality RNA, these samples are further diluted according to directions in the protocol. Diluting these samples accordingly eliminates problems in the extraction procedure.
8. Can I use water to elute the RNA?
No. The elution buffer supplied in the kit must be used
9. As part of my RT procedure I routinely heat an aliquot of the eluate prior to the RT reaction. Do I still need to heat the whole eluate after the RNA preparation?
Yes. Heating temperature and incubation time will vary depending on different RT protocols. Therefore, the final heating step should be performed routinely at the end of the RNA purification protocol.
10. Is a A260/A230 nm absorbance ratio specified for RNA derived from the PAXgene Bone Marrow RNA System?
No. An A260/A230 nm absorbance ratio is not specified for any PAXgene Bone Marrow RNA Kit. In contrast to a reduced A260/A280 ratio, which results from protein contamination of the eluted RNA and can lead to inhibitory effects in downstream applications, there is no negative impact of a reduced A260/A230 ratio on any downstream application reported to date and therefore, it is not necessary to specify this.
In addition, because the elution buffer contains very low concentrations of salt that absorb at wavelengths between 220 and 230 nm, it is necessary to zero the photometer properly. Do this by preparing a solution for zeroing the photometer which contains a similar salt concentration as the sample to be measured by using the same volumes. Add the same volume of Buffer BMR5 (as the volume of RNA sample to be diluted) into a fresh volume of the buffer in which you will measure the absorption (we recommend 10 mM Tris Cl pH 7.5 buffer for this purpose).
11. Can the PAXgene RNA stabilization solution/bone marrow mixture from the PAXgene Bone Marrow RNA Tube be disposed of by adding bleach?
Yes. You may dispose of the waste product from the PAXgene Bone Marrow RNA Tube with a 5% sodium hypochlorite bleach solution, in a 1:9 ratio (1 part bleach : 9 parts tube contents).
12. How are the supernatants resulting from the first two spin steps of the purification procedure disposed since they still may contain biologically hazardous material?
We recommend classifying the tube-processed supernatant as both a biological and chemical waste. Supernatants can be decontaminated by autoclaving and disposed of as chemical waste.
CAUTION: Do not add bleach or acidic solutions directly to the sample preparation waste. Disposal should be done in accordance with local, state, and federal regulations.
13. What is proper disposal procedure for waste from the sample preparation?
Waste from the sample preparation, such as supernatants from centrifugation steps in the RNA purification procedure, is to be considered potentially infectious. Before disposal, the waste must be autoclaved or incinerated to destroy any infectious material. Disposal must follow official regulations.

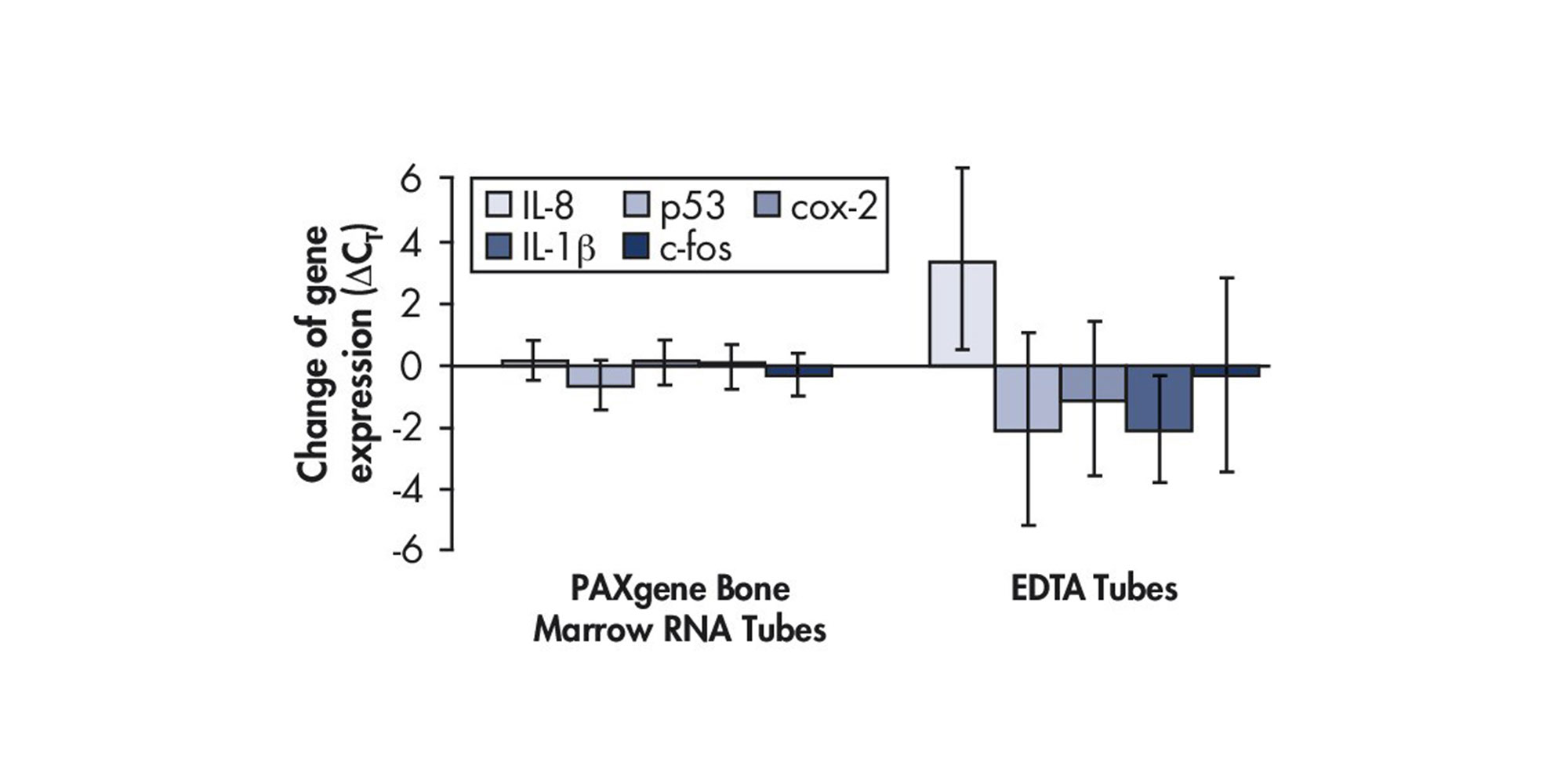
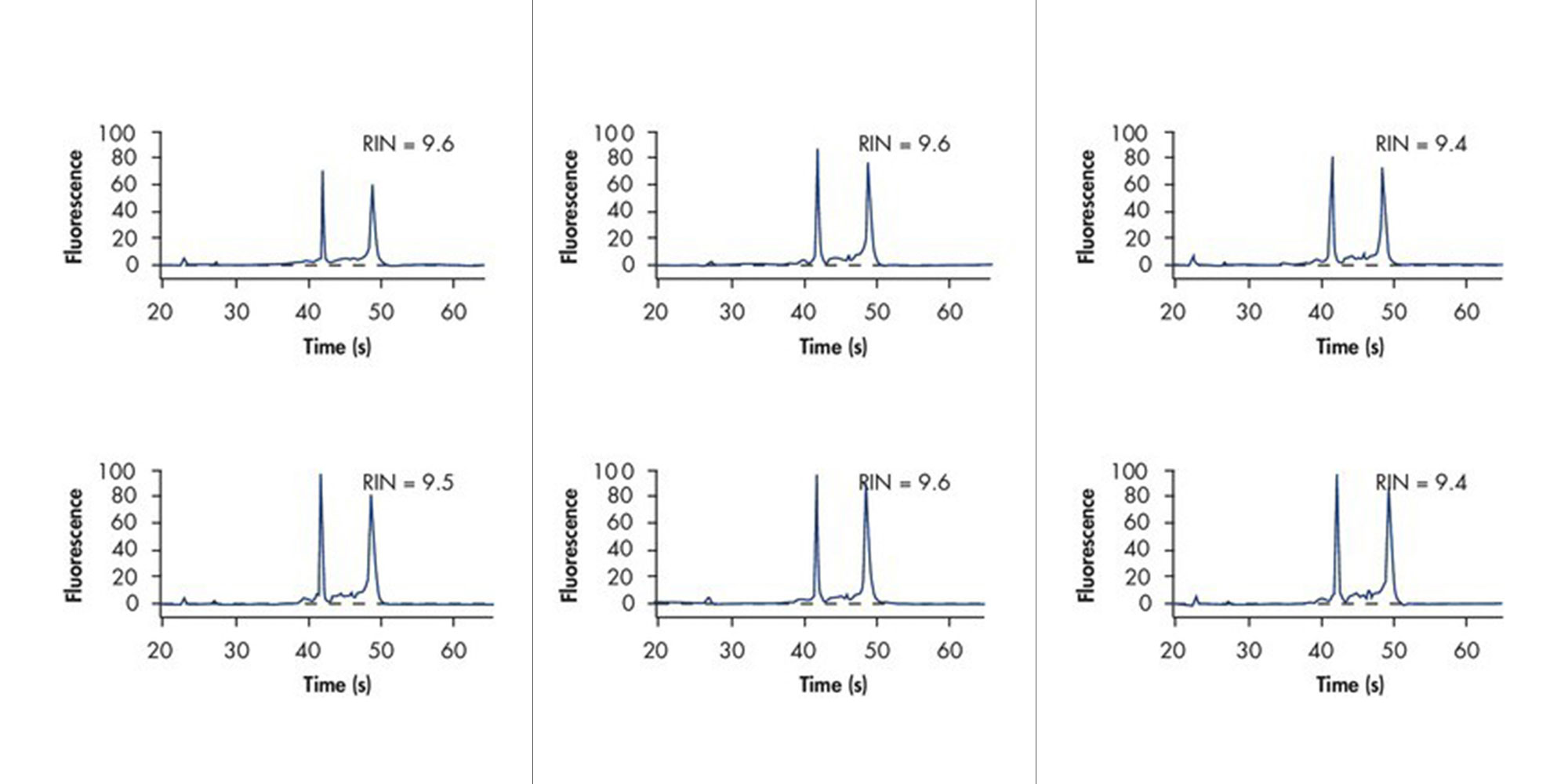
![Figure 6. RNA stability at room temperature. Bone marrow samples were collected from 39 individuals and immediately transferred to [A] PAXgene Bone Marrow RNA Tubes or [B] EDTA tubes. RNA was purified using the PAXgene Bone Marrow RNA Kit or a reference silica-membrane-based method at day 0 or after 2 days storage at room temperature. Expression levels of five (5) marker transcripts for each sample (IL-8, p53, cox-2, IL-1β, c-fos) were quantified using real-time RT-PCR. The high correlation of CT values (threshold cycles) for the samples stabilized in PAXgene Bone Marrow RNA Tubes indicates that the levels of these transcripts did not significantly change during storage, in contrast to the samples stored in EDTA tubes.](/storage/images/Content/Product_pages/Bone_Marrow_RNA/RNA-stability-at-room-temperature.jpg)
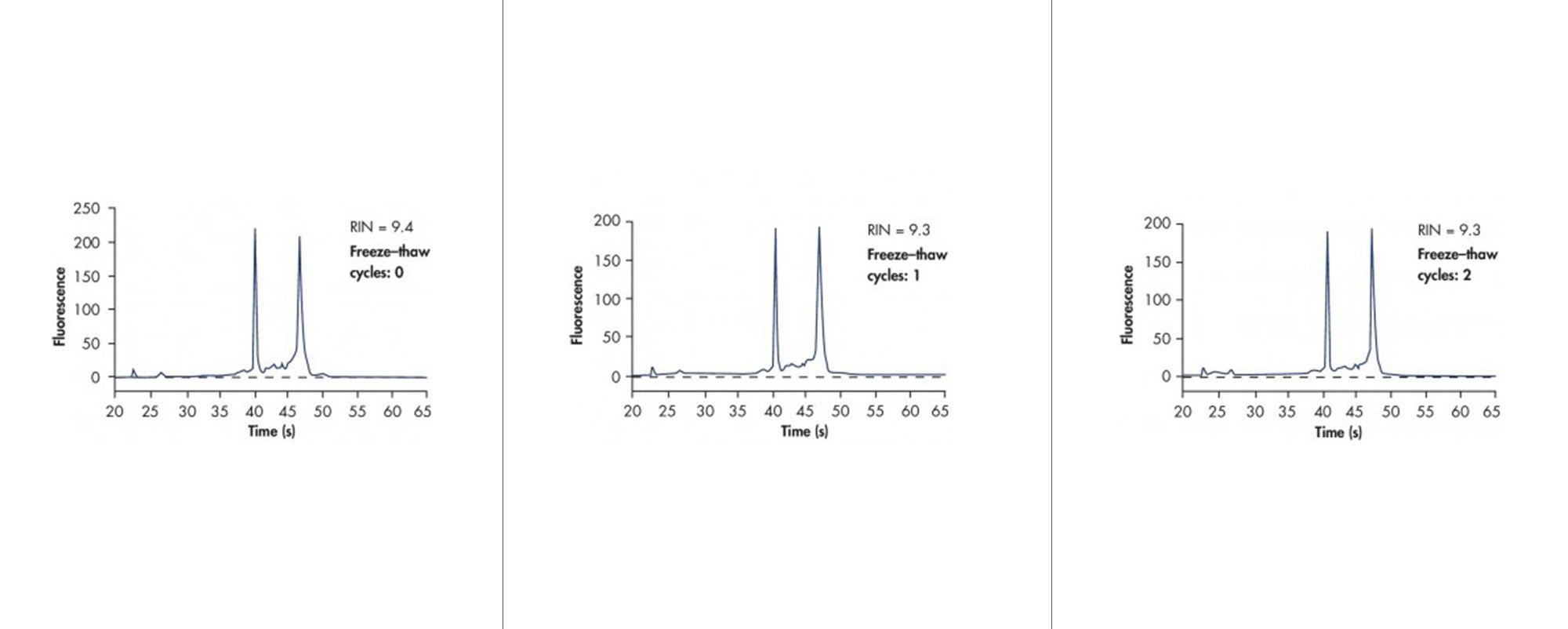
![Figure 7. RNA purity for samples stored under different conditions. Bone Marrow samples were stored in PAXgene Bone Marrow RNA Tubes [A] for 3 days at 18-25°C, [B] for 5 days at 2-8°C, or [C] subjected to 2 freeze-thaw cycles. RNA was purified using the PAXgene Bone Marrow RNA Kit, and RNA purity was analyzed by absorbance. The A260/A280 ratios indicate highly pure RNA from samples stored under different conditions.](/storage/images/Content/Product_pages/Bone_Marrow_RNA/RNA-Purity-for-samples-stored-under-different-conditions.jpg)