Performance
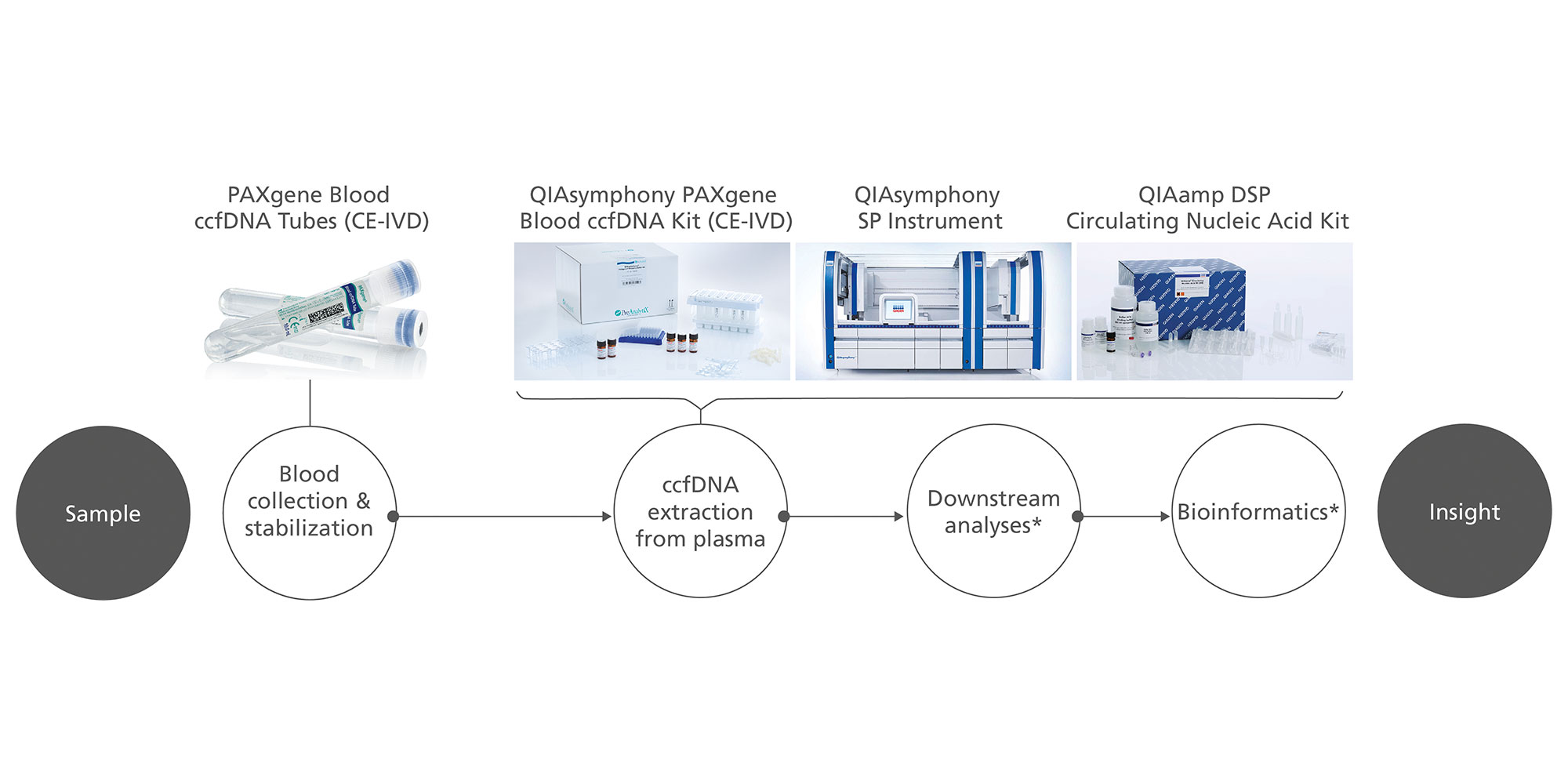
The QIAsymphony PAXgene Blood ccfDNA workflow (CE-IVD) consists of a blood collection tube, the PAXgene Blood ccfDNA Tube (CE-IVD), and a circulating cell-free DNA (ccfDNA) purification kit, the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD), for automated extraction of ccfDNA on the QIAsymphony SP instrument. Together, the tube and kit constitute a complete in vitro diagnostic medical device preanalytical workflow (Figure 1).
The QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) magnetic-particle technology enables purification of high-quality ccfDNA that is free of proteins, nucleases, and other impurities.
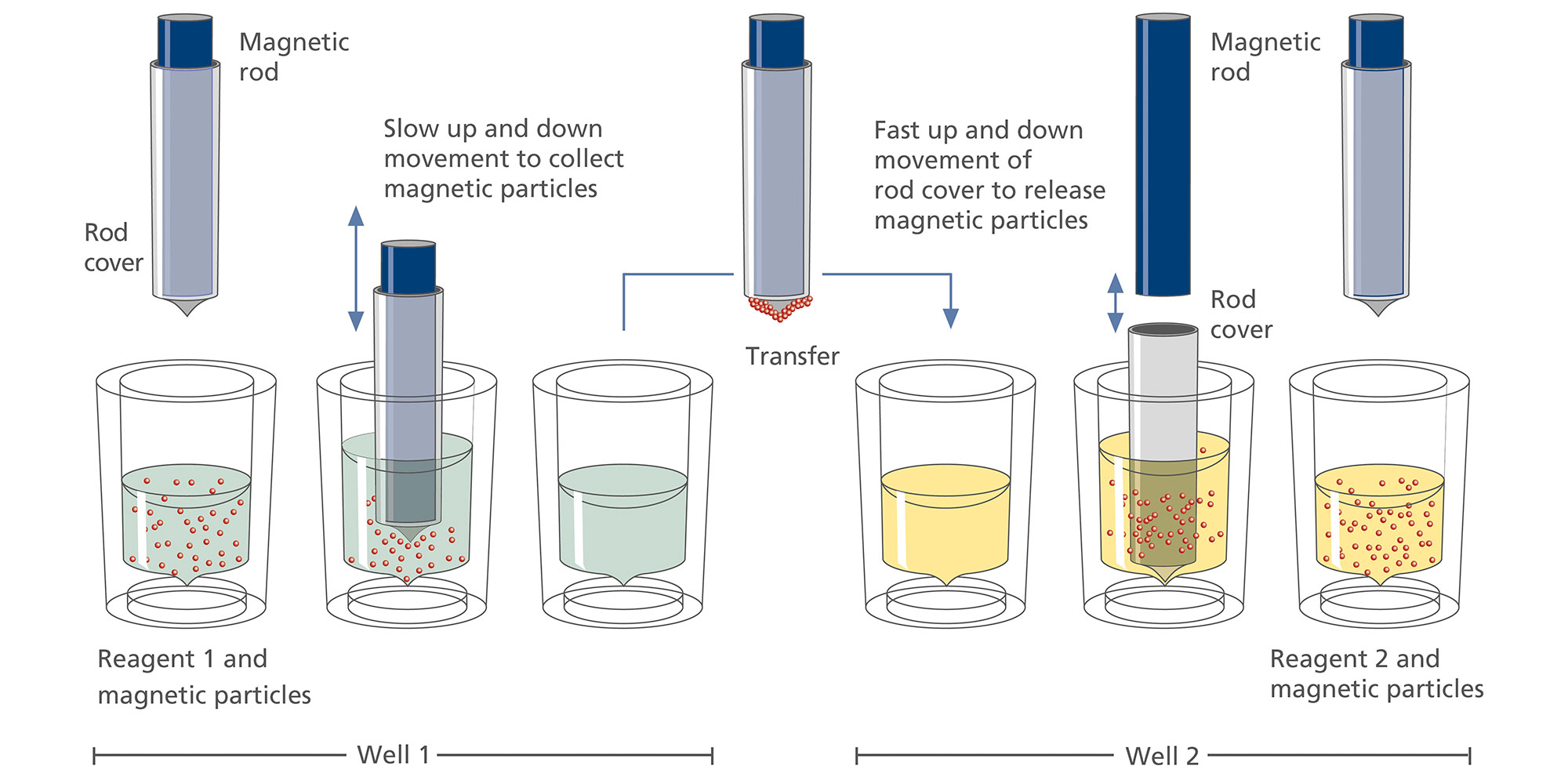
The QIAsymphony SP instrument performs all steps of the purification procedure. The technology combines the speed and efficiency of anion exchange-based nucleic acid purification with the convenient handling of magnetic particles. The purification procedure is designed to ensure safe and reproducible handling of potentially infectious samples. Onboard measures minimize cross-contamination. Up to 96 samples, in batches of 24, are processed in a single run (Figure 2).
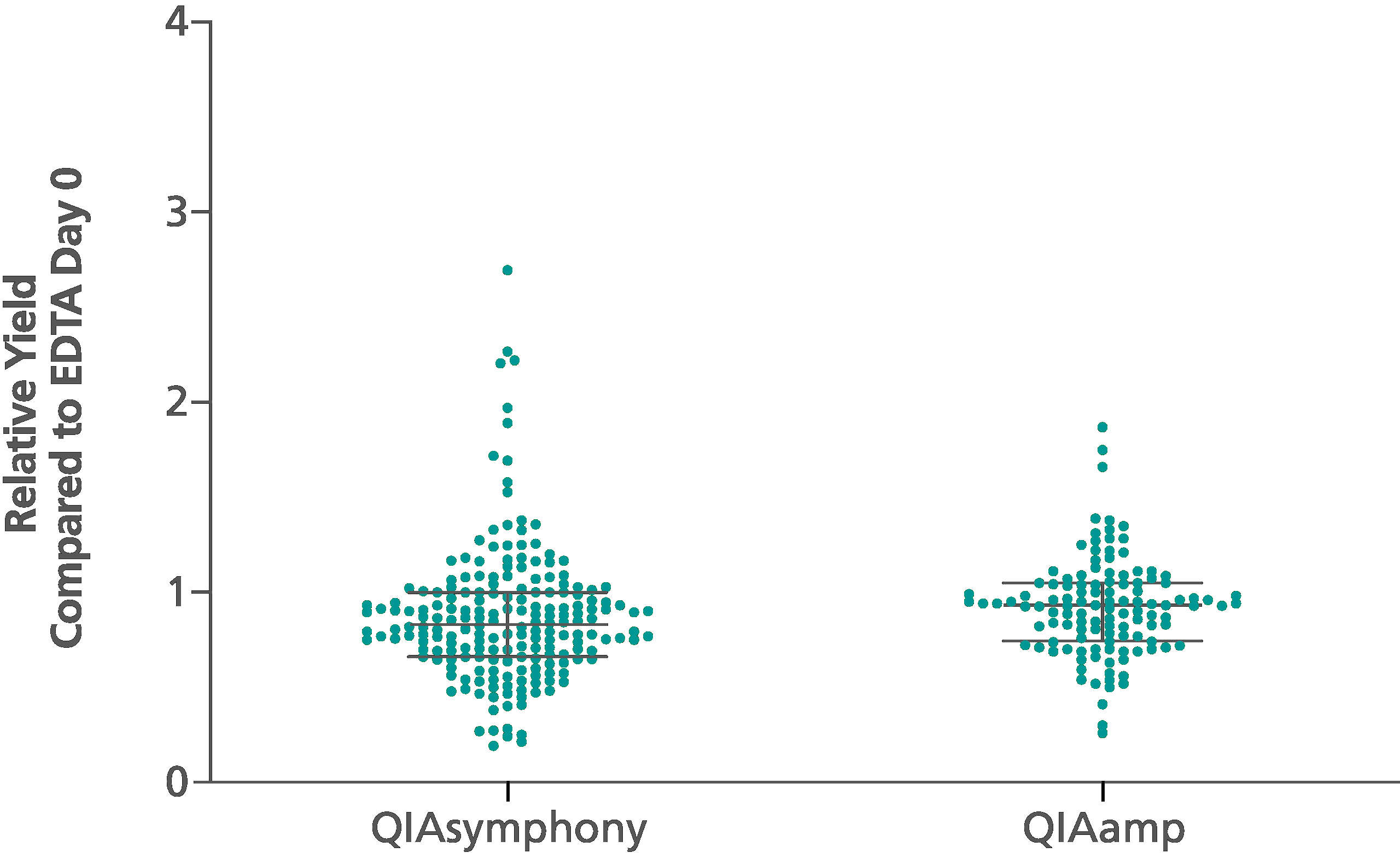
If similar input volumes of blood are used, ccfDNA yields achieved with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) are comparable to manual extraction with the QIAamp DSP Circulating Nucleic Acid Kit (Figure 3).
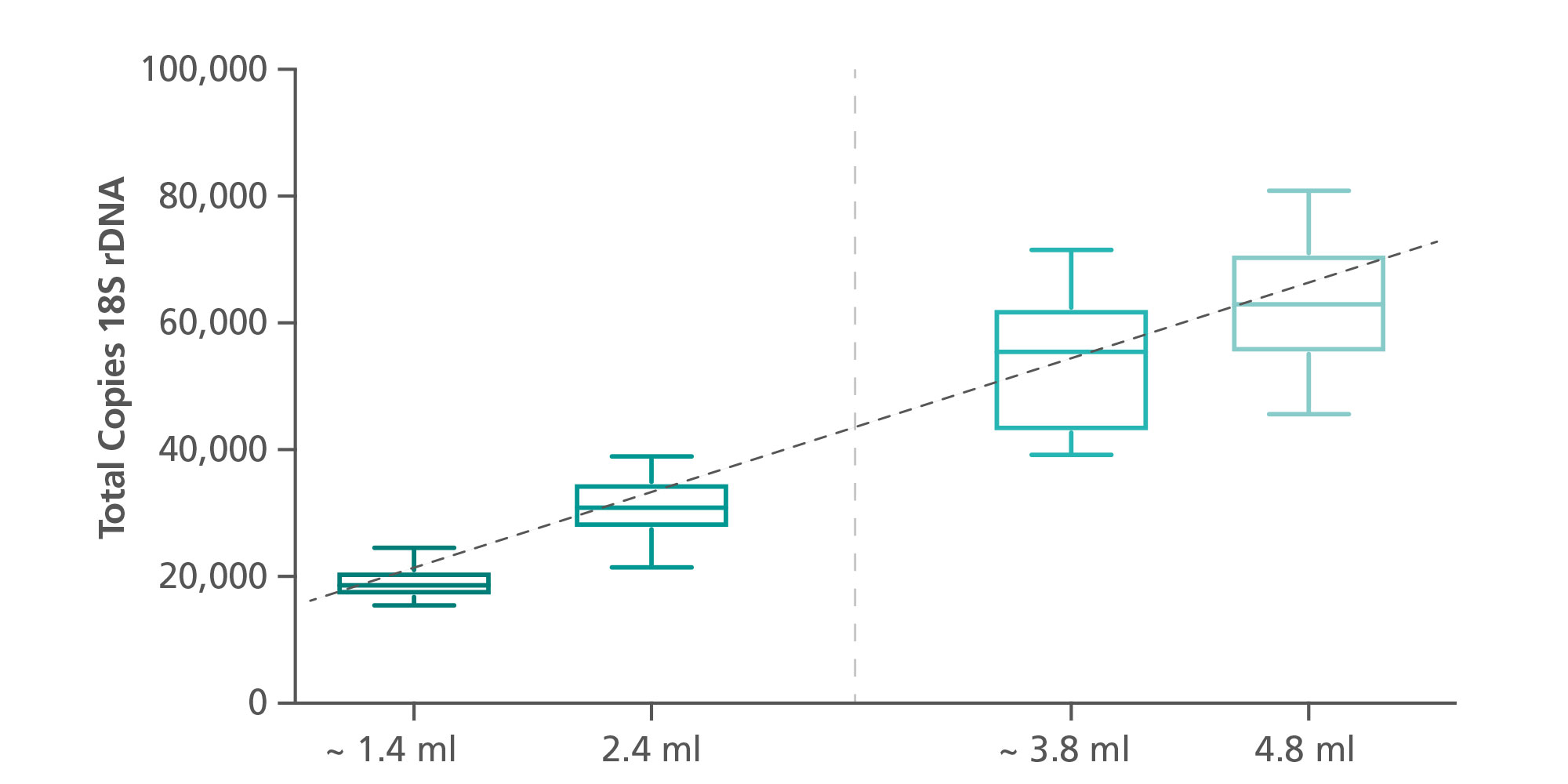
Four different protocols were established. In the standard versions, sample input volumes of 2.4 mL or 4.8 mL plasma can be selected. A “less sample” function, an integrated part of the protocol, allows the transfer of lower plasma volumes with minimum input volumes of 1.6 mL or 4.1 mL, respectively (Figure 4).
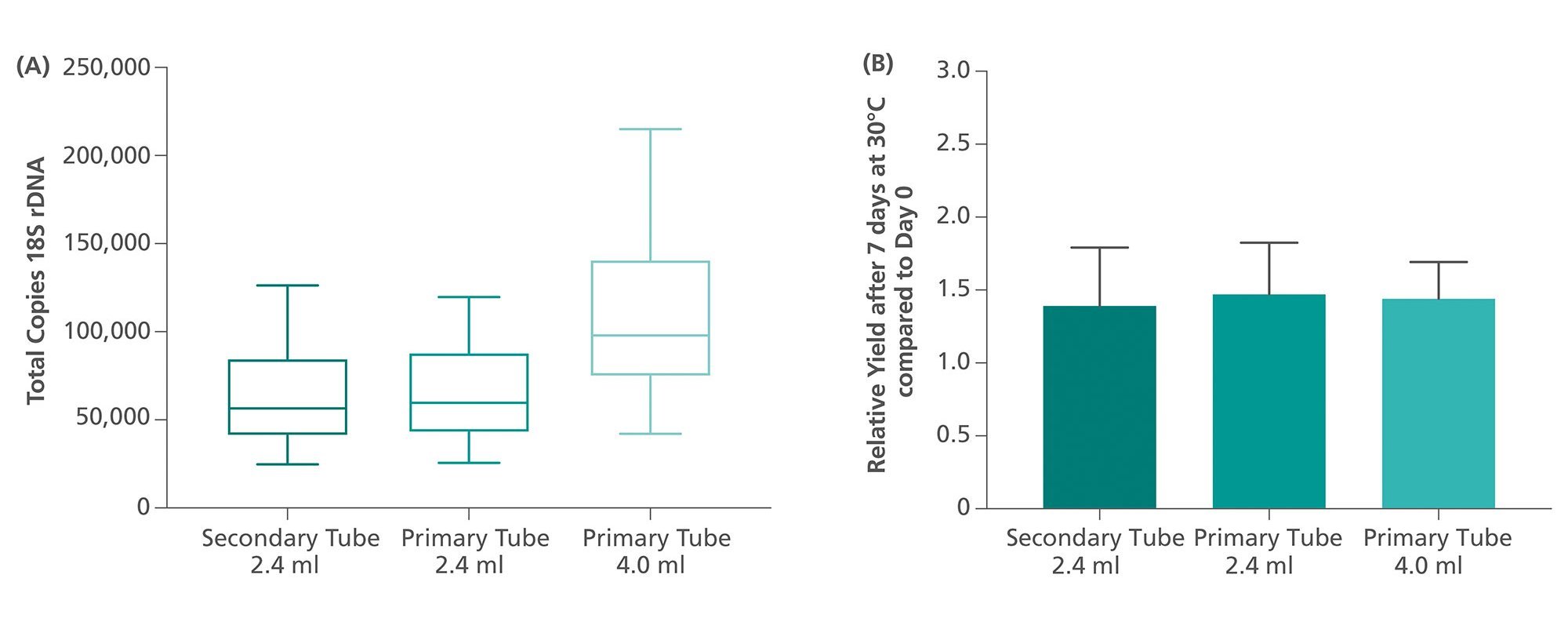
The Primary Tube handling protocols allow direct placement of the PAXgene Blood ccfDNA Tube (CE-IVD) onto the QIAsymphony SP instrument. Primary handling saves time, reduces costs and biohazard waste, and minimizes misidentification and risk of exposure. Primary Tube handling protocols are available for sample input volumes of 2.4 mL or 4.0 mL plasma (Figure 5).
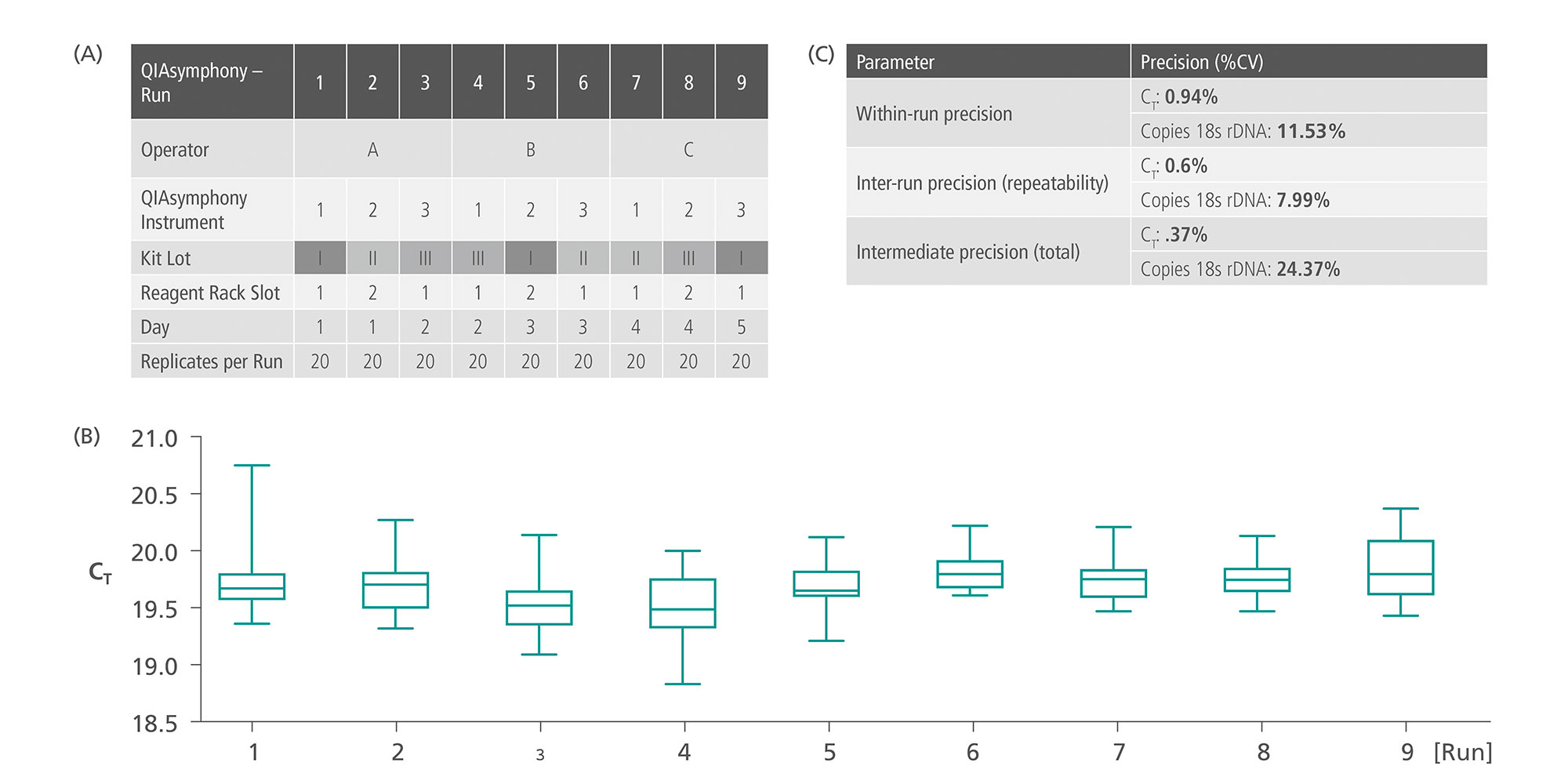
Preanalytical steps including handling, storage, processing, and documentation of verification and validation studies of QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) were conducted in accordance with ISO 20186-3:2019: Molecular in vitro diagnostic examinations — Specifications for pre-examination processes for venous whole blood — Part 3: Isolated circulating cell free DNA from plasma. The QIAsymphony PAXgene Blood ccfDNA workflow (CE-IVD) covers the whole liquid biopsy preanalytical workflow from blood collection to automated ccfDNA extraction from plasma with high intermediate, inter-run and within-run precision (Figure 6).
The purified ccfDNA eluates can be stored for long-term at −20°C or −80°C, including at least three freeze and thaw cycles (for latest update see technical note ´Eluate Stability Study´) and are compatible for downstream analytical PCR assays, including user-validated methylation-based PCR, and next-generation sequencing (NGS) molecular test methods.
Principle
Circulating cell-free DNA (ccfDNA) is present in plasma usually as short fragments (<1000 bp). The concentration of ccfDNA in plasma is usually low (can range from 1–100 ng/ml) and varies considerably between individuals.
The PAXgene Blood ccfDNA Tube (CE-IVD) is a plastic, closed, evacuated tube for the collection, anticoagulation, transport, and storage of 10 ml human whole blood samples. The additive in the Tube prevents the release of intracellular DNA into the plasma and maintains constant ccfDNA levels for 10 days at temperatures up to 25°C, 7 days at temperatures up to 30°C, or 3 days at temperatures up to 37°C.
Plasma generated from whole blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) can be used to process ccfDNA automated with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) on the QIAsymphony SP instrument.
The PAXgene Blood ccfDNA Tube (CE-IVD) with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) and QIAGEN QIAsymphony instrument (CE-IVD) have been validated as an integrated workflow.
Procedure
Blood is collected under a standard phlebotomy protocol into the PAXgene Blood ccfDNA Tube (see PAXgene Blood ccfDNA Tube (CE-IVD) Instructions for Use).
In the standard protocols, plasma is generated by centrifugation at room temperature (15–25°C) for 15 minutes at 1,600–3,000 × g. Plasma is removed after the first centrifugation into a secondary tube, centrifuged again for 10 minutes at 1,600-3,000 × g, transferred into a 14 mL 17 × 100 mm polystyrene, round-bottom tube, placed into a tube carrier, and loaded into the sample input drawer of the QIAsymphony SP instrument.
Sample input volumes of 2.4 mL or 4.8 mL plasma can be selected, but due to void volumes of an additional 0.4 mL and 0.5 mL, a minimum of 2.8 mL and 5.3 mL plasma respectively must be placed onto the instrument. If lower plasma volumes than 2.8 mL or 5.3 mL are available, the “Less Sample mode”, an integrated part of the protocol function, allows processing of lower plasma volumes by the instrument. In the result file, the discrepancy between the regular and the transferred plasma volume is documented. The minimum plasma input volumes to enable “Less Sample mode” are 1.6 mL or 4.1 mL.
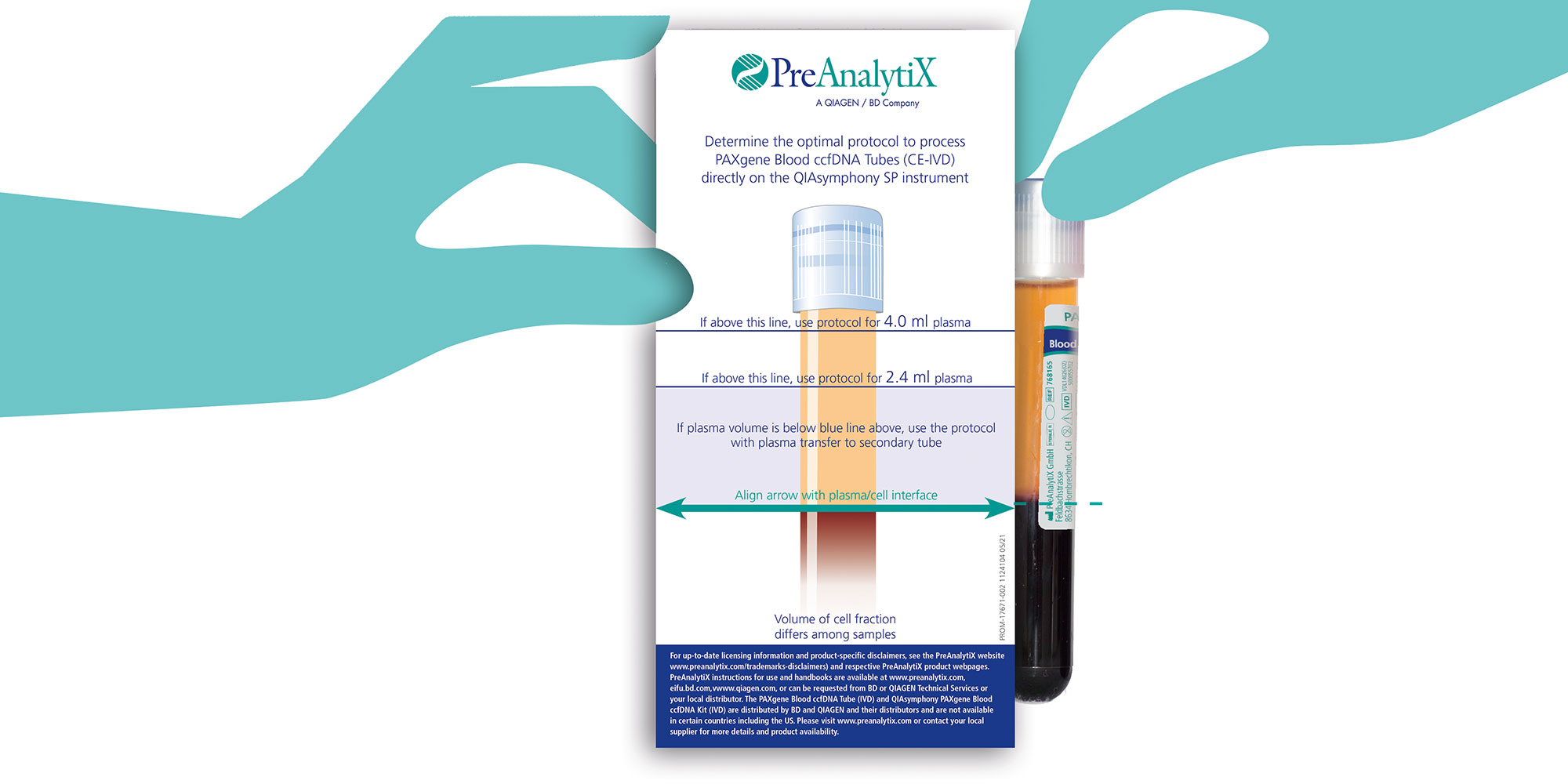
As an alternative to the standard protocols, the primary tube handling function allows the tube to be directly placed on the QIAsymphony SP instrument after the first centrifugation. In order to use the primary tube handling function, centrifugation for 15 minutes at 3,000 × g is mandatory. Plasma volume in each tube after removal from the centrifuge bucket are conveniently quantified with the PAXgene Blood ccfDNA Purification Protocol Selection Tool provided as a kit content to select one of the Primary Tube handling protocols for sample input volumes of 2.4 mL or 4.0 mL plasma (Figure 7).
On the QIAsymphony SP instrument, plasma proteins are digested by proteinase K while ccfDNA binds to the surface of magnetic beads. Three wash steps ensure contaminant removal.
Finally, ccfDNA is eluted from the magnetic particles and is ready for use in downstream IVD applications.
Applications
ccfDNA purified from plasma generated from whole blood collected in PAXgene Blood ccfDNA Tubes (CE-IVD) using the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) on the QIAsymphony SP instrument is ready for use in a wide range of downstream applications, including:
- PCR, including digital, multiplex and quantitative real-time PCR
- Pharmacogenomic studies
- SNP genotyping
- Next-generation sequencing
- User-validated methylation assays
PAXgene Blood ccfDNA Tube (CE-IVD) and QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) are for in vitro diagnostic use.