Performance
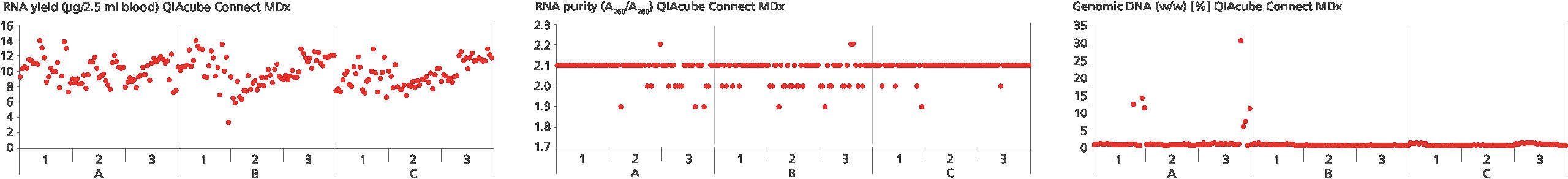
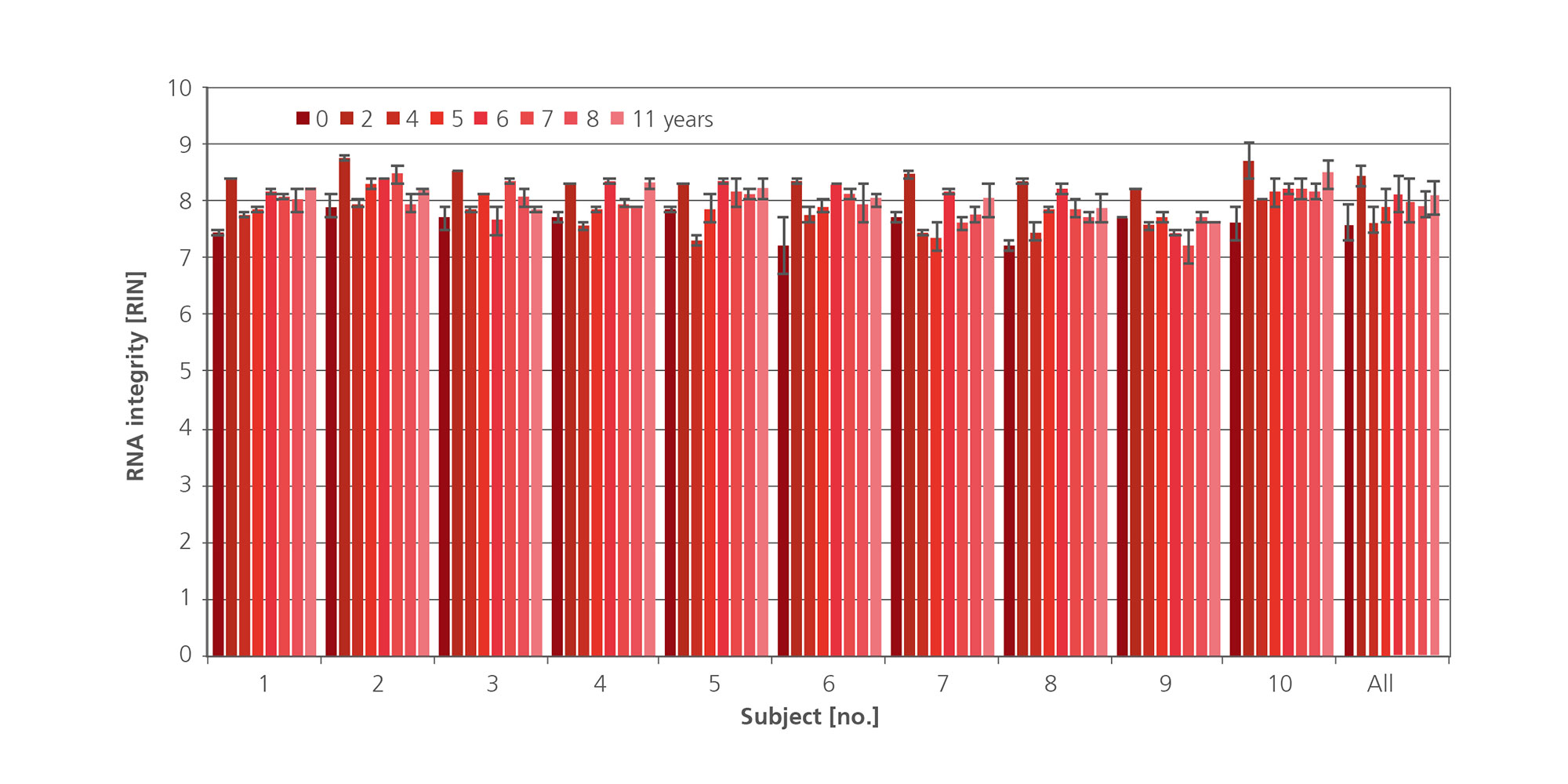
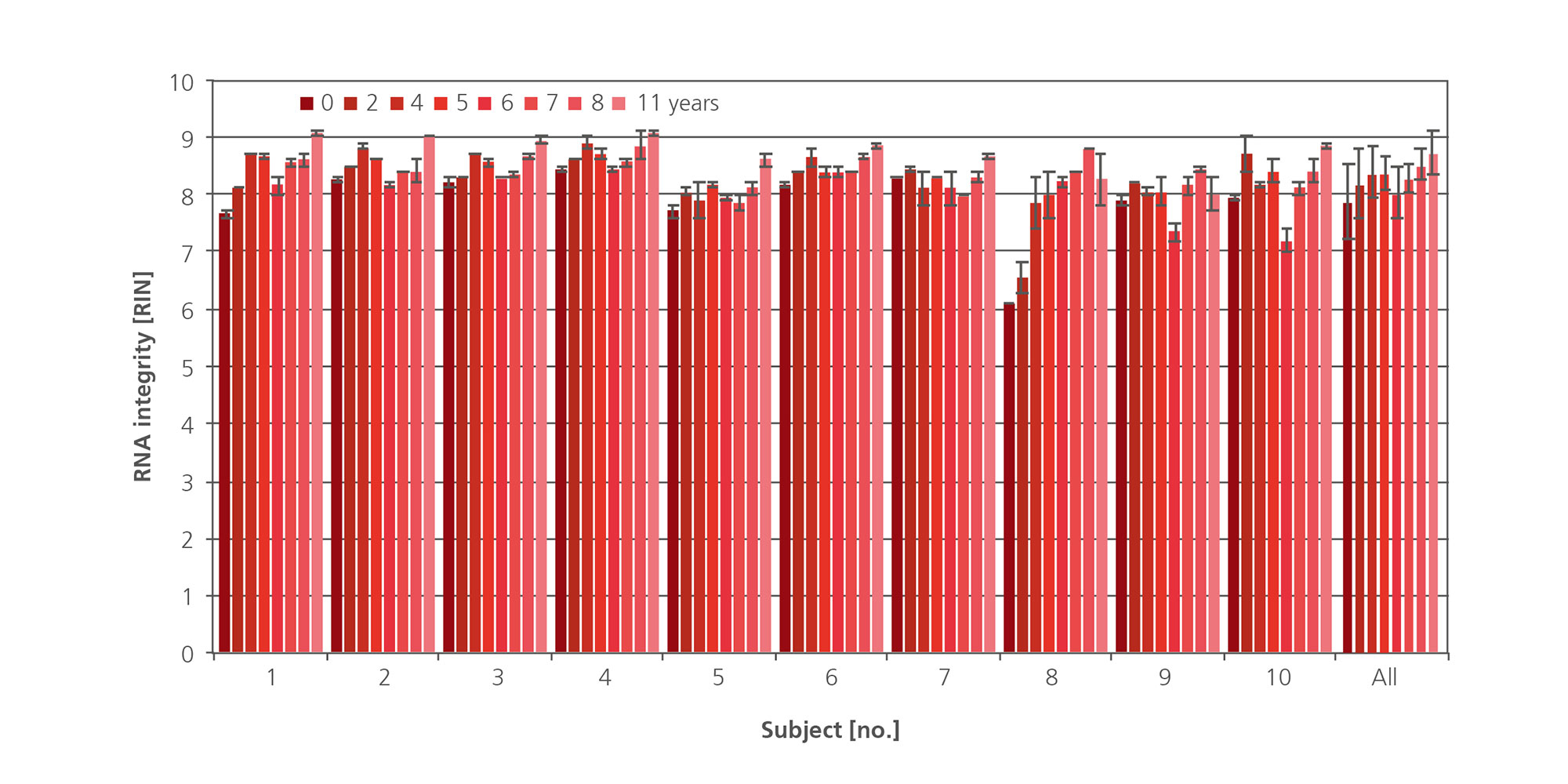
The additive in PAXgene Blood RNA Tubes protects RNA molecules from degradation by RNases and other enzymes and triggers the complete lysis of all cells in the sample to minimize ex vivo changes in gene expression due to gene induction and downregulation. The result is immediate and allows long-term stabilization of RNA for up to 3 days at room temperature (Figure 1 and Figure 2) or refrigerated for up to 5 days (Figure 3 and Figure 4). Current data shows stabilization of intracellular RNA for up to 11 years at –20°C or –70°C (see Technical Notes).
Principle
The collection of whole blood is the first step in many molecular assays used to study intracellular RNA. A major challenge in this type of testing is the instability of intracellular RNA, which rapidly degrades within hours after blood collection. Furthermore, certain species of RNA, through the process of gene induction, increase in vitro after blood collection. Both in vitro RNA degradation and gene induction can lead to an under or overestimation of in vivo relative gene transcript number.
The PAXgene Blood RNA Tube contains an additive that stabilizes the in vivo gene transcription profile by reducing in vitro RNA degradation and minimizing gene induction. When used in conjunction with one of the PAXgene Blood RNA Kits, the PAXgene Blood RNA Tube provides pure RNA for accurate detection and quantification of gene expression.
Procedure
Whole blood is collected directly into the PAXgene Blood RNA Tube, using standard phlebotomy practice and equipment. The RNA in the specimen is stabilized for storage at room temperature, refrigerated, or frozen. RNA can be purified using one of the PAXgene Blood RNA Kits (FDA and CE). The ease of collection, stabilization of RNA at different temperatures, and elimination of additional processing steps, such as centrifugation at the collection facility and storage on ice prior to RNA purification, contributes to standardize the workflow from collection to purification, reducing variability that can be introduced by different operators (Figure 5).
Applications
When used in conjunction with the PAXgene Blood RNA Kit, the PAXgene Blood RNA Tubes enable purification of intracellular RNA from whole blood for applications in molecular diagnostic testing, such as real-time RT-PCR analysis of specific transcripts.
PAXgene Blood RNA Tubes are intended for in vitro diagnostic testing (IVD) and are to be used in conjunction with the PAXgene Blood RNA Kit.

![Figure 1. RNA stability at 18–25°C: FOS. Blood was drawn from ten apparently healthy consented donors, with duplicate samples, and stored at 18–25°C for the indicated number of days (x-axis), followed by total RNA purification. [A] Blood was collected and stored in PAXgene Blood RNA Tubes, and total RNA was purified using the PAXgene Blood RNA Kit. [B] Blood was collected and stored in standard blood collection tubes with EDTA as an anticoagulant, and total RNA was purified using a standard organic-extraction method with silica-membrane–based RNA cleanup. Relative transcript levels of FOS were determined by real-time, duplex RT-PCR, using 18S rRNA as an internal standard. The values for all samples are plotted, with means and standard deviations of all samples shown. The dashed lines indicate the ±3x total precision of the assay (2.34 CT).](/storage/images/_processed_/b/9/csm_RNA_stability_at_18_25C_FOS_4bfc9d7fcc.jpg)
![Figure 2. RNA stability at 18–25°C: IL1B. Blood was drawn from ten apparently healthy consented donors, with duplicate samples, and stored at 18–25°C for the indicated number of days (x-axis), followed by total RNA purification. [A] Blood was collected and stored in PAXgene Blood RNA Tubes, and total RNA was purified using the PAXgene Blood RNA Kit. [B] Blood was collected and stored in standard blood collection tubes with EDTA as an anticoagulant, and total RNA was purified using a standard organic-extraction method with silica-membrane–based RNA cleanup. Relative transcript levels of IL1B were determined by real-time, duplex RT-PCR, using 18S rRNA as an internal standard. The values for all samples are plotted, with means and standard deviations of all samples shown. The dashed lines indicate the ±3x total precision of the assay (1.93 CT).](/storage/images/_processed_/0/3/csm_RNA_stability_at_18.25_C_IL_18_cfbacacdcb.jpg)
![Figure 3. RNA stability at 2–8°C: FOS. Blood was drawn from ten apparently healthy consented donors, with duplicate samples, and stored at 2–8°C for the indicated number of days (x-axis), followed by total RNA purification. [A] Blood was collected and stored in PAXgene Blood RNA Tubes, and total RNA was purified using the PAXgene Blood RNA Kit. [B] Blood was collected and stored in standard blood collection tubes with EDTA as an anticoagulant, and total RNA was purified using a standard organic-extraction method with silica-membrane-based RNA cleanup. Relative transcript levels of FOS were determined by real-time, duplex RT-PCR, using 18S rRNA as an internal standard. The values for all samples are plotted, with means and standard deviations of all samples shown. The dashed lines indicate the ±3x total precision of the assay (2.34 CT).](/storage/images/Content/Product_pages/Blood/RNA_miRNA/RNA_stability_at_2-8_C_FOS.jpg)
![Figure 4. RNA stability at 2–8°C: IL1B. Blood was drawn from ten apparently healthy consented donors, with duplicate samples, and stored at 2–8°C for the indicated number of days (x-axis), followed by total RNA purification. [A] Blood was collected and stored in PAXgene Blood RNA Tubes, and total RNA was purified using the PAXgene Blood RNA Kit. [B] Blood was collected and stored in standard blood collection tubes with EDTA as an anticoagulant, and total RNA was purified using a standard organic-extraction method with silica-membrane-based RNA cleanup. Relative transcript levels of IL1B were determined by real-time, duplex RT-PCR, using 18S rRNA as an internal standard. The values for all samples are plotted, with means and standard deviations of all samples shown. The dashed lines indicate the ±3x total precision of the assay (1.93 CT).](/storage/images/Content/Product_pages/Blood/RNA_miRNA/RNA_stability_at_2-8_C_IL_1B.jpg)
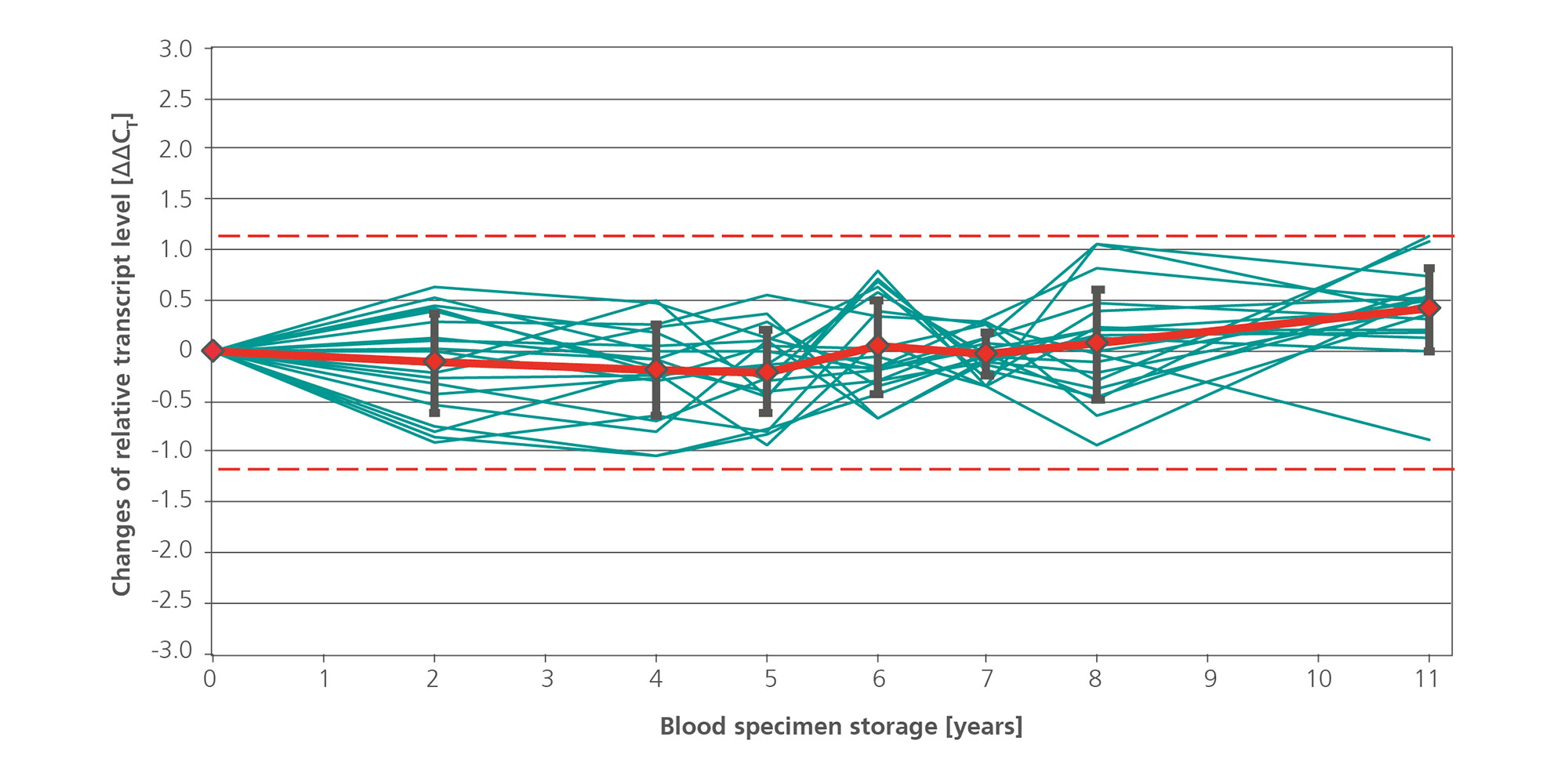
![Figure 5. Reproducibility between users. Blood samples from 30 different apparently healthy consented donors were collected in PAXgene Blood RNA Tubes (12 tubes per donor, 360 tubes in total). The contents of the tubes from 3 donors were pooled and subsequently realiquoted into 36 samples. These 36 samples per 3-donor-pool were manually processed by 3 different operators. Each operator used 3 different PAXgene Blood RNA Kit lots for the extraction and processed quadruplicate samples from each of the 10 donor pools. Relative transcript levels of [A] FOS and [B] IL1B were determined by real-time, duplex RT-PCR using 18S rRNA as an internal standard. The values for all samples are plotted, relative to the values for user 1 (10 donor pools x 3 kit lots x 4 replicates = 120 data sets for each gene), with means (red lines) and standard deviations (black bars) for all samples shown. The dashed lines indicate the ±3x total precision of the assays (FOS, 2.34 CT; IL1B, 1.93 CT).](/storage/images/_processed_/2/c/csm_Reproducibility_between_users_8142f519f9.jpg)


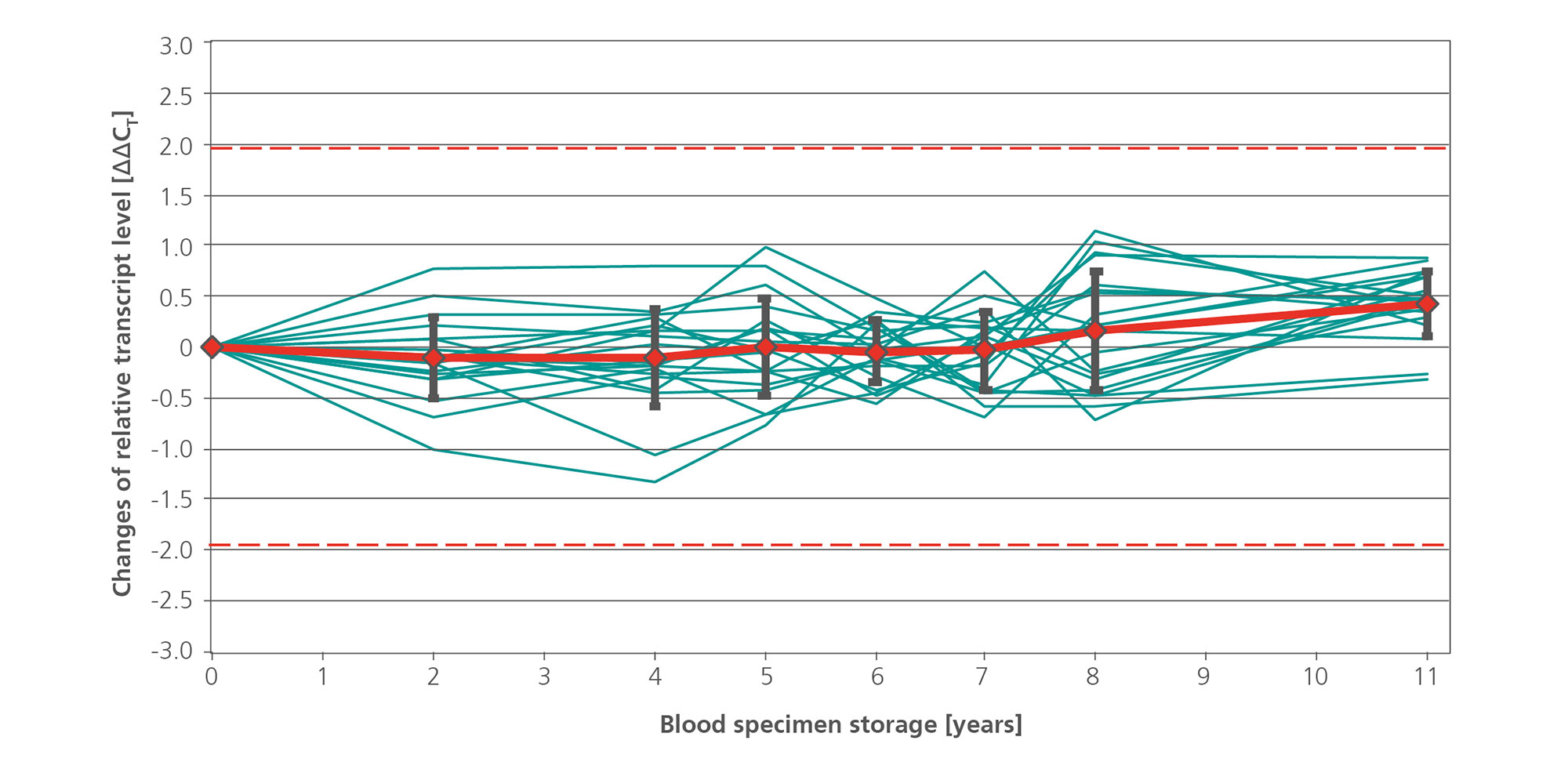
![Figure 12. Reproducibility between kit lots. Blood samples from 30 different apparently healthy consented donors were collected in PAXgene Blood RNA Tubes (12 tubes per donor, 360 tubes in total). The contents of the tubes from 3 donors were pooled and subsequently realiquoted into 36 samples. These 36 samples per 3-donor-pool were manually processed by 3 different operators. Each operator used 3 different PAXgene Blood RNA Kit lots for the extraction and processed quadruplicate samples from each of the 10 donor pools. Relative transcript levels of [A] FOS and [B] IL1B were determined by real-time, duplex RT-PCR using 18S rRNA as an internal standard. The values for all samples are plotted, relative to the values for kit lot 1 (10 donor pools x 3 users x 4 replicates = 120 data sets for each gene), with means (red lines) and standard deviations (black bars) for all samples shown. The dashed lines indicate the ±3x total precision of the assays (FOS, 2.34 CT; IL1B, 1.93 CT).](/storage/images/Content/Product_pages/Blood/RNA_miRNA/Reproducibility_between_kit_lots.jpg)