Performance
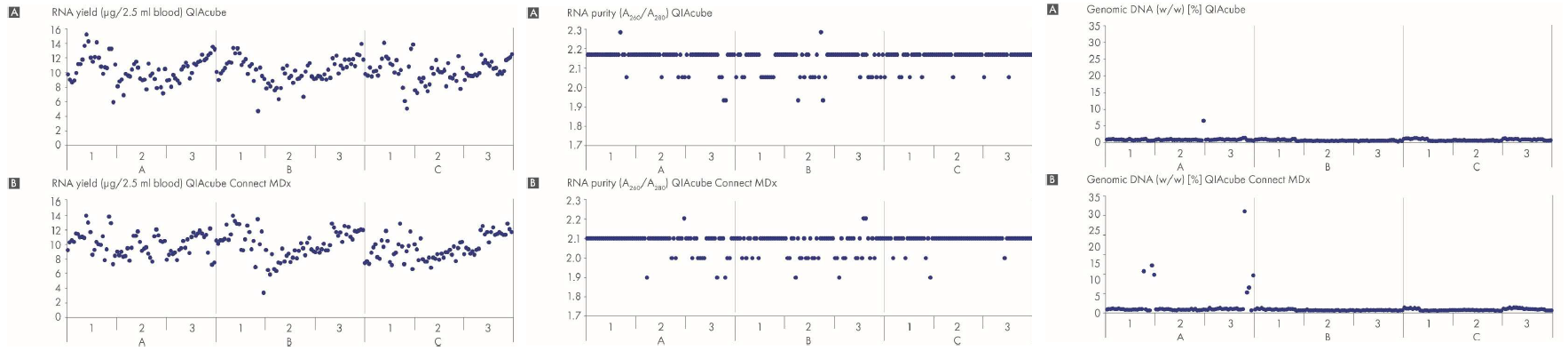
Total RNA purified using the PAXgene Blood RNA System is highly pure, with A260/A280 values between 1.8 and 2.2 and ≤1.0% (w/w) genomic DNA in ≥95% of all samples processed (Figure 1). At least 95% of samples show no inhibition in RT-PCR, when using up to 30% of the eluate. RNA yields from 2.5 mL healthy human whole blood are ≥3 µg for ≥95% of the samples processed. Since yields are highly donor-dependent, individual yields may vary. For individual donors, the PAXgene Blood RNA System provides highly reproducible and repeatable yields (Figure 2 and Figure 3), making the System highly robust for clinical diagnostic tests.
The automated protocol of RNA purification using the PAXgene Blood RNA System provides pure RNA in high yield with reproducible and repeatable RT-PCR results, as shown in the figures (Figure 1 and Figure 4). Statistics of RT-PCR results are summarized in the table below. Cross-contamination between samples is undetectable, as measured by quantitative, real-time RT-PCR of sequences of the betaglobin and FOS transcripts in RNA-negative samples (water) paired with RNA-positive samples (human whole blood) in the same run.
Table 1.Summary statistics of RT-PCR results using automated protocol
Test system | FOS/18S rRNA assay | IL1B/18S rRNA assay | ||
|---|---|---|---|---|
Comparison of data | Mean (ΔΔCT) | ± SD (ΔΔCT) | Mean (ΔΔCT) | ± SD (ΔΔCT) |
Reproducibility within each user and between all lots | ||||
All users, lot 1 – lot 1 | 0.00 | 0.00 | 0.00 | 0.00 |
All users, lot 1 – lot 2 | –0.03 | 0.48 | –0.07 | 0.66 |
All users, log 1 – lot 3 | –0.21 | 0.52 | 0.11 | 0.71 |
Reproducibility within each lot and between all users | ||||
All lots, user A – user A | 0.00 | 0.00 | 0.00 | 0.00 |
All lots, user A – user B | –0.46 | 0.44 | –0.06 | 0.69 |
All lots, user A – user C | –0.31 | 0.60 | –0.15 | 0.71 |
User: Technician performed the study.
Lot: Number of kit lot used in this study.
SD: Standard deviation.
Principle
Copy numbers of individual RNA species in blood can change significantly during storage or transport at ambient temperature, making reliable studies of gene expression difficult. Both stabilization of RNA molecules after collection and an efficient RNA purification protocol are needed to maximize insights into expression profiles of whole blood. Based on silica-membrane technology in spin column format, and when combined with PAXgene Blood RNA Tubes, the PAXgene Blood RNA Kit provides a rapid procedure and the chemistry to isolate RNA of high quality and purity.
Procedure
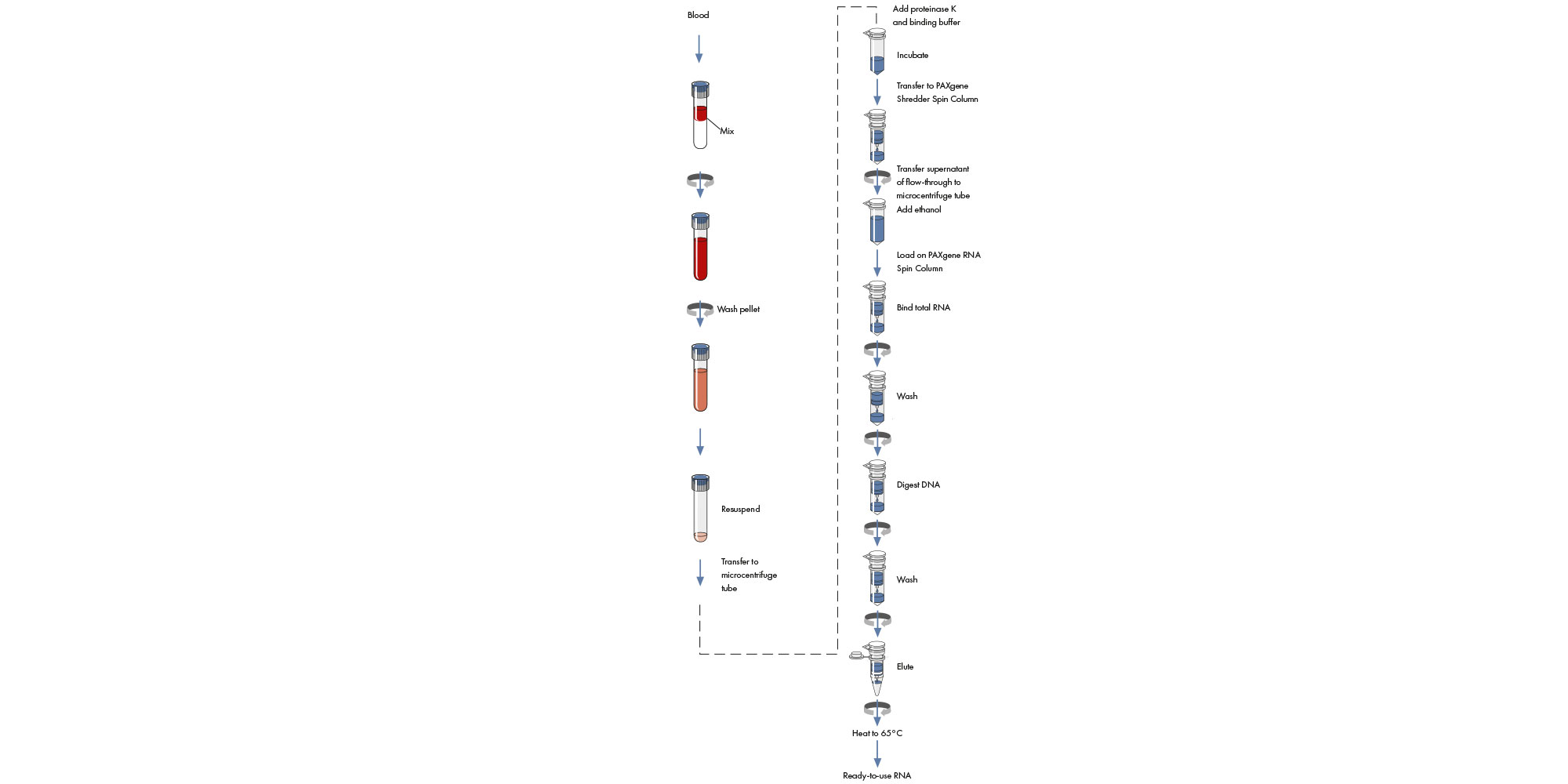
The PAXgene Blood RNA procedure is simple and can be performed using manual or automated procedures (Figure 5 and Figure 6).
Manual PAXgene Blood RNA procedure
The PAXgene Blood RNA procedure begins with a centrifugation step to pellet nucleic acids in the PAXgene Blood RNA Tube. The pellet is washed, resuspended, and incubated in optimized buffers together with proteinase K to bring about protein digestion. An additional centrifugation through the PAXgene Shredder spin column is carried out to homogenize the cell lysate and remove residual cell debris, and the supernatant of the flow-through fraction is transferred to a fresh microcentrifuge tube. Ethanol is added to adjust binding conditions, and the lysate is applied to a PAXgene RNA spin column. During a brief centrifugation, RNA is selectively bound to the PAXgene silica membrane as contaminants pass through. Remaining contaminants are removed in several efficient wash steps. Between the first and second wash steps, the membrane is treated with DNase I to remove trace amounts of bound DNA. After the wash steps, RNA is eluted in elution buffer and heat-denatured.
Automated PAXgene Blood RNA procedure
Sample preparation, automated on the QIAcube Connect MDx, follows the same steps as the manual procedure, enabling you to continue using the PAXgene Blood RNA Kit for purification of high-quality RNA.
The precedure begins with a centrifugation step to pellet nucleic acids in the PAXgene Blood RNA Tube. The pellet is washed, resuspended, and transferred from the PAXgene Blood RNA Tube into processing tubes, which are placed into the thermoshaker unit on the QIAcube Connect MDx worktable. The operator selects and starts the "PAXgene Blood RNA Part A" protocol from the menu. The QIAcube Connect MDx performs the steps of the protocol through to elution of RNA in elution buffer. The operator transfers the microcentrifuge tubes, containing the purified RNA, into the thermoshaker unit of the QIAcube Connect MDx. The operator selects and starts the "PAXgene Blood RNA Part B" protocol from the menu, and heat denaturation is performed by the QIAcube Connect MDx. Average sample preparation time (based on data from 12 sample preps) is 151 minutes, with only approximately 20 minutes of hands-on time.
Applications
When the kit is used in conjunction with PAXgene Blood RNA Tubes, the system provides intracellular RNA from whole blood for RT-PCR used in molecular diagnostic testing.
The PAXgene Blood RNA Kit is for in vitro diagnostics (IVD).
![Figure 2. Repeatability and reproducibility of RNA yield. Blood samples from 30 different donors were collected in PAXgene Blood RNA Tubes (12 tubes per donor, 360 tubes in total). The contents of the tubes from 3 donors were pooled and subsequently realiquoted into 36 samples. These 36 samples per 3-donor-pool were manually processed by 3 different operators. Each operator used 3 different PAXgene Blood RNA Kit lots for the extraction and processed quadruplicate samples from each of the 10 donor pools. [A] RNA yield and standard deviation for every operator–lot combination. Quadruplicate blood samples from 10 donor pools were processed by 3 different operators (A, B, C) with each of 3 kit lots (1, 2, 3). The mean yields (columns) and standard deviations (error bars) per quadruplicate sample from the same donor pool for different operator and different kit lot are presented. [B] CV of RNA yield per donor pool for all operator–lot combinations (A, B, C; 1, 2, 3) as calculated from the mean yield and standard deviation of the yield shown in part A.](/storage/images/Content/Product_pages/Blood/RNA_miRNA/Repeatability_and_reproducibility_of_RNA_yield_0.jpg)
![Figure 3. Reproducible and repeatable RNA purification. Quadruplicate blood samples from 14 donors were manually processed by each of 3 technicians (A, B, C). Three sets of equipment were used, and all samples prepared by a single technician were processed using the same equipment. [A] Means and standard deviations of RNA yield per replicate samples from the same donors and different technicians are shown. [B] Twelve replicate blood samples from each of 14 donors were processed by the 3 different technicians. Means and standard deviations of RNA yield per samples from the same donors and all technicians are presented. For all RNA samples, A260/A280 ratios ranged from 1.8 to 2.2.](/storage/images/Content/Product_pages/Blood/RNA_miRNA/Reproducible_and_repeatable_RNA_purification.png)
![Figure 4. Reproducibility between automated and manual protocols. RNA was purified by 3 different operators (A, B, C) using 3 different lots (1, 2, 3) of the PAXgene Blood RNA Kit using the automated protocol in the experiment described in the figure "RNA yield and purity — automated processing". In parallel, RNA was purified from the corresponding replicate tubes using the manual protocol. Relative transcript levels of [A] FOS and [B] IL1B were determined by real-time, duplex RT-PCR using 18S rRNA as an internal standard. Possible differences of transcript levels between RNA prepared from paired blood samples using both extraction protocols (automated and manual protocol) were calculated by the ΔΔCT method. Individual ΔΔCT values for all sample pairs (4 replicates x 6 donor pools x 3 kit lots x 3 operators = 216 pairs for each gene) are plotted as single dots with means) are plotted as single dots with means (larger dots) and standard deviations (black bars) for all samples shown. The dashed lines indicate the ±3x total precision of the assays (FOS: 1.16 CT; IL1B: 1.98 CT)](/storage/images/Content/Product_pages/Blood/RNA_miRNA/Reproducibility_between_automated_and_manual_protocols.png)