Performance
PAXgene Blood ccfDNA Tubes (CE-IVD) simplify the collection and processing of whole blood for subsequent purification of circulating, cell-free DNA (ccfDNA) from plasma and genomic DNA (gDNA) from the nucleated cellular fraction. The tube additive is non-crosslinking, free of formaldehyde-releasing substances and inhibits apoptosis.
Using PAXgene Blood ccfDNA Tubes (CE-IVD) for whole blood collection, storage and transport helps prevent the release of intracellular DNA into plasma (Figure 1).
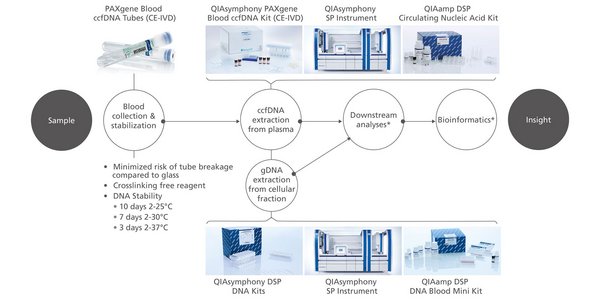
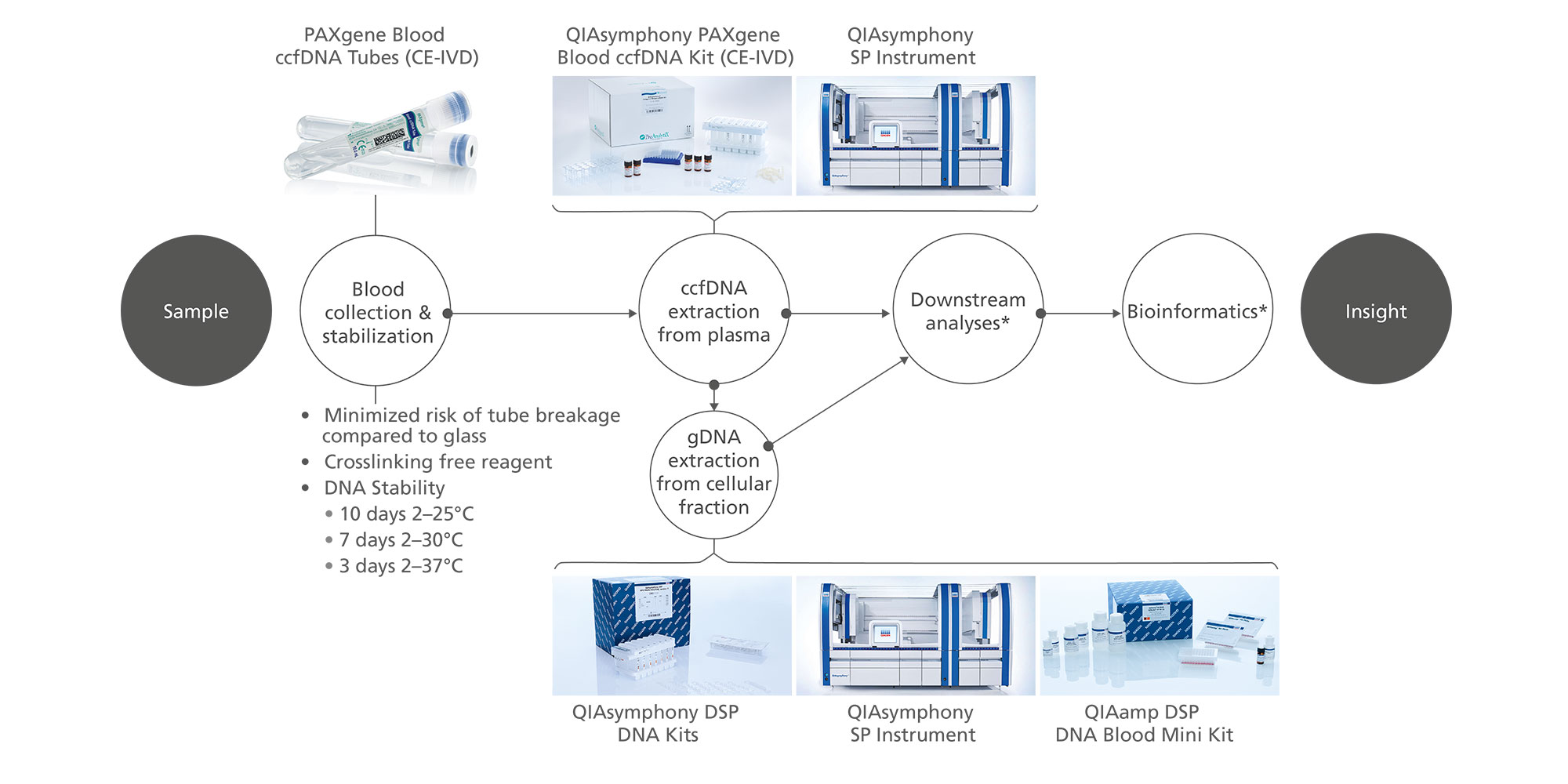
Plasma generated from whole blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) can be used to process ccfDNA automated with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) or manually with the QIAamp DSP Circulating Nucleic Acid Kit. The nucleated cellular fraction or buffy coat remaining after removal of the plasma can be processed for gDNA automated with the QIAsymphony DSP DNA Mini and Midi Kits, or manually with the QIAamp DSP DNA Blood Mini Kit (Figure 2).
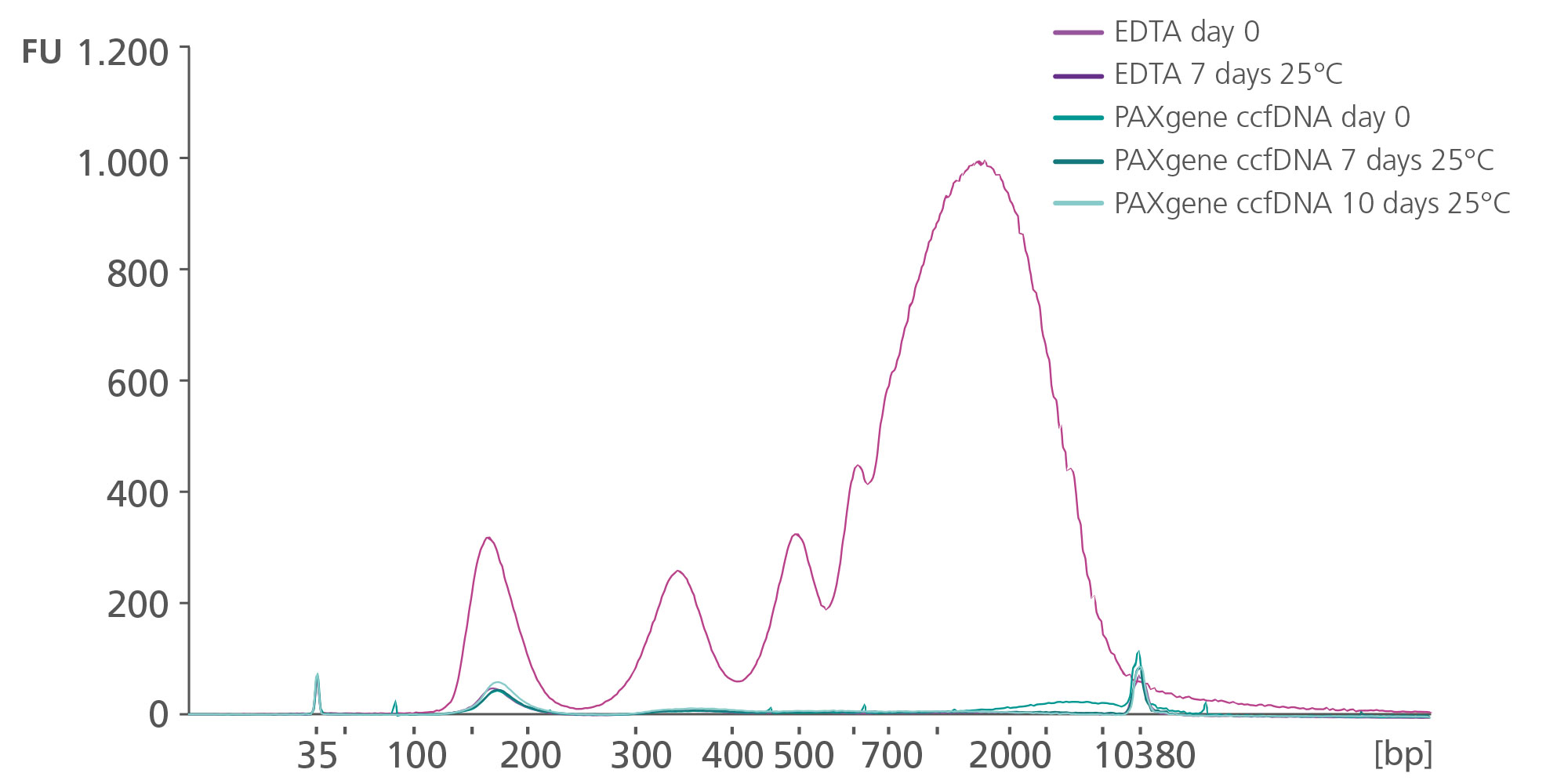
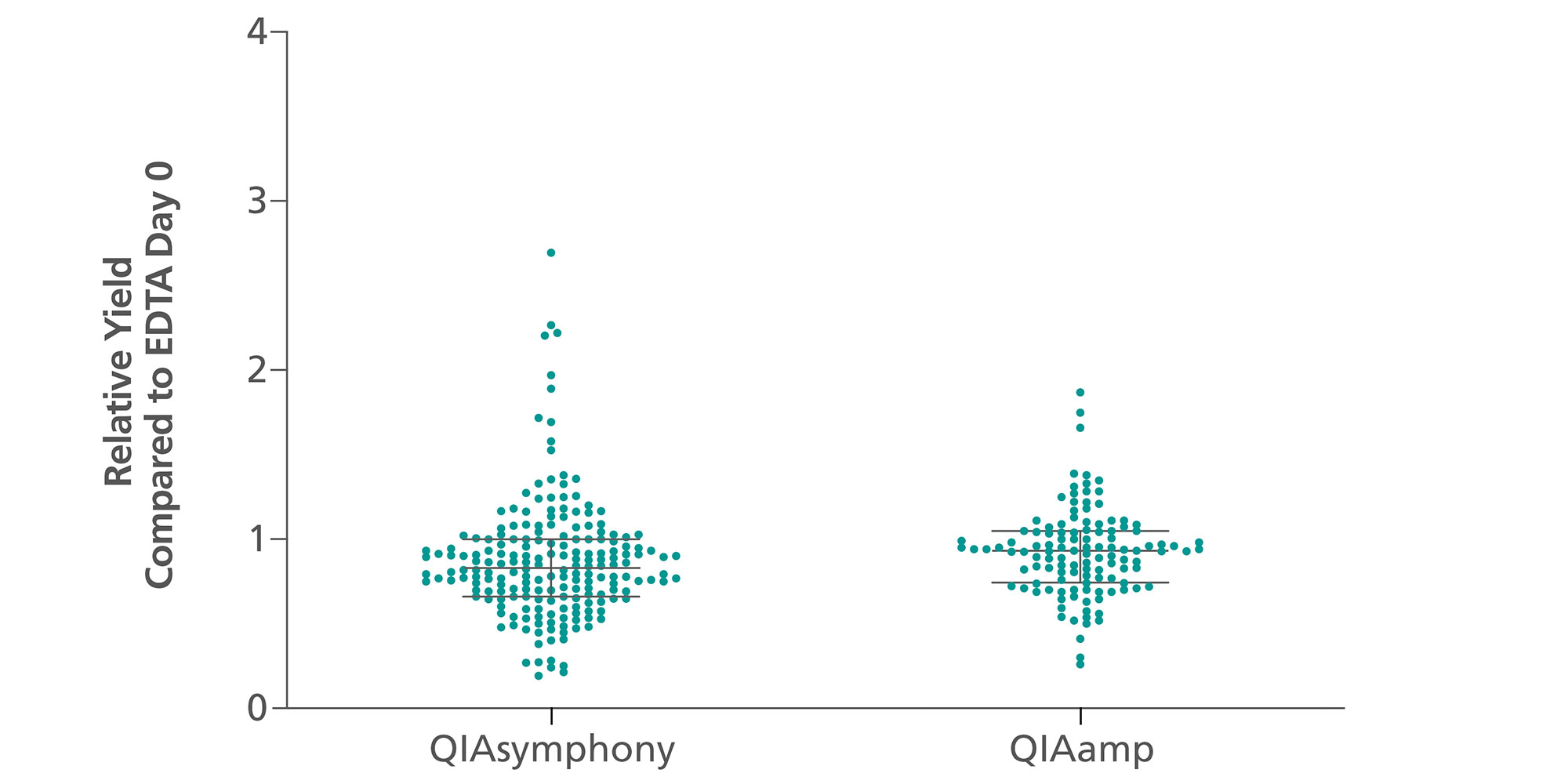
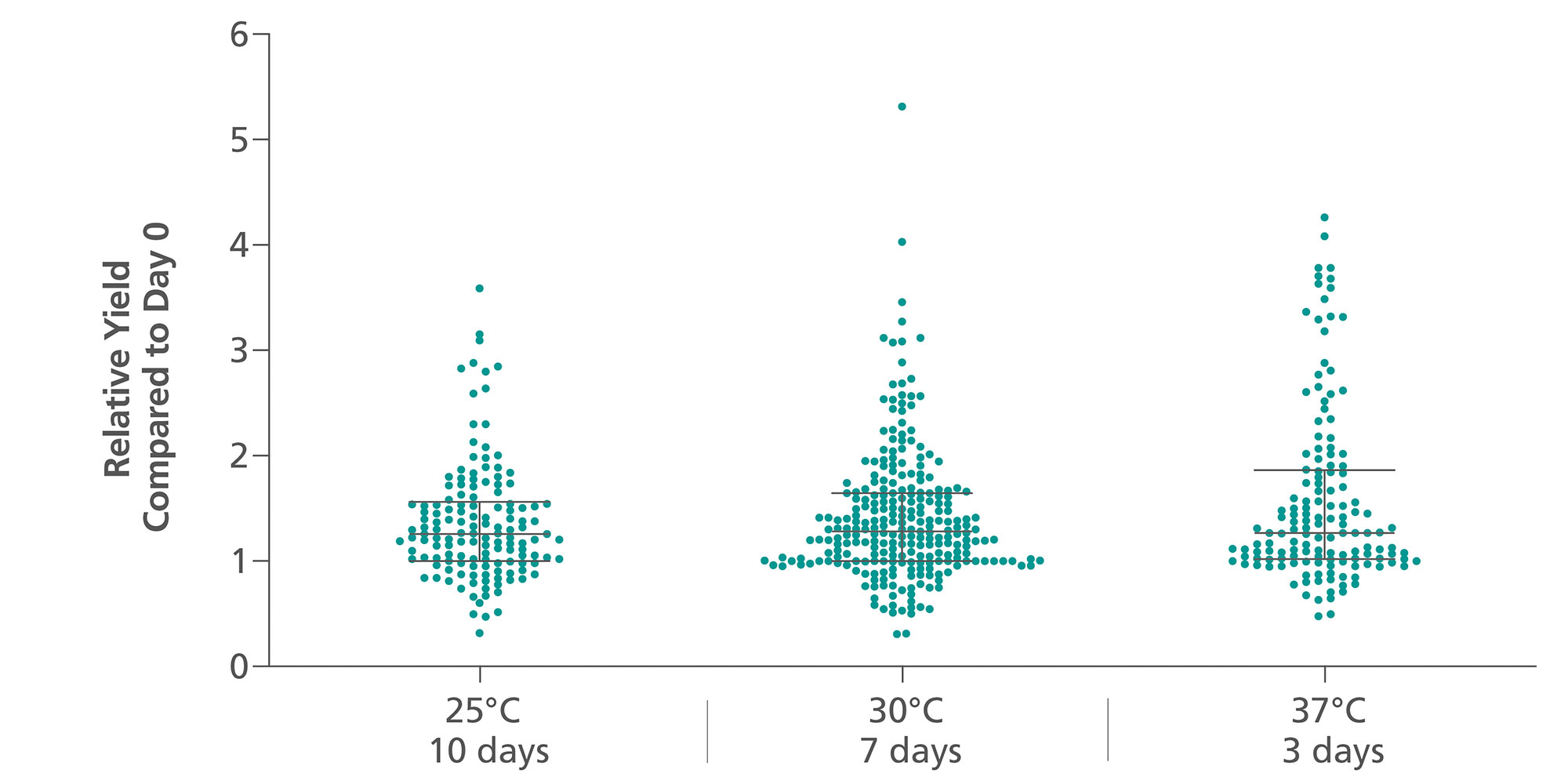
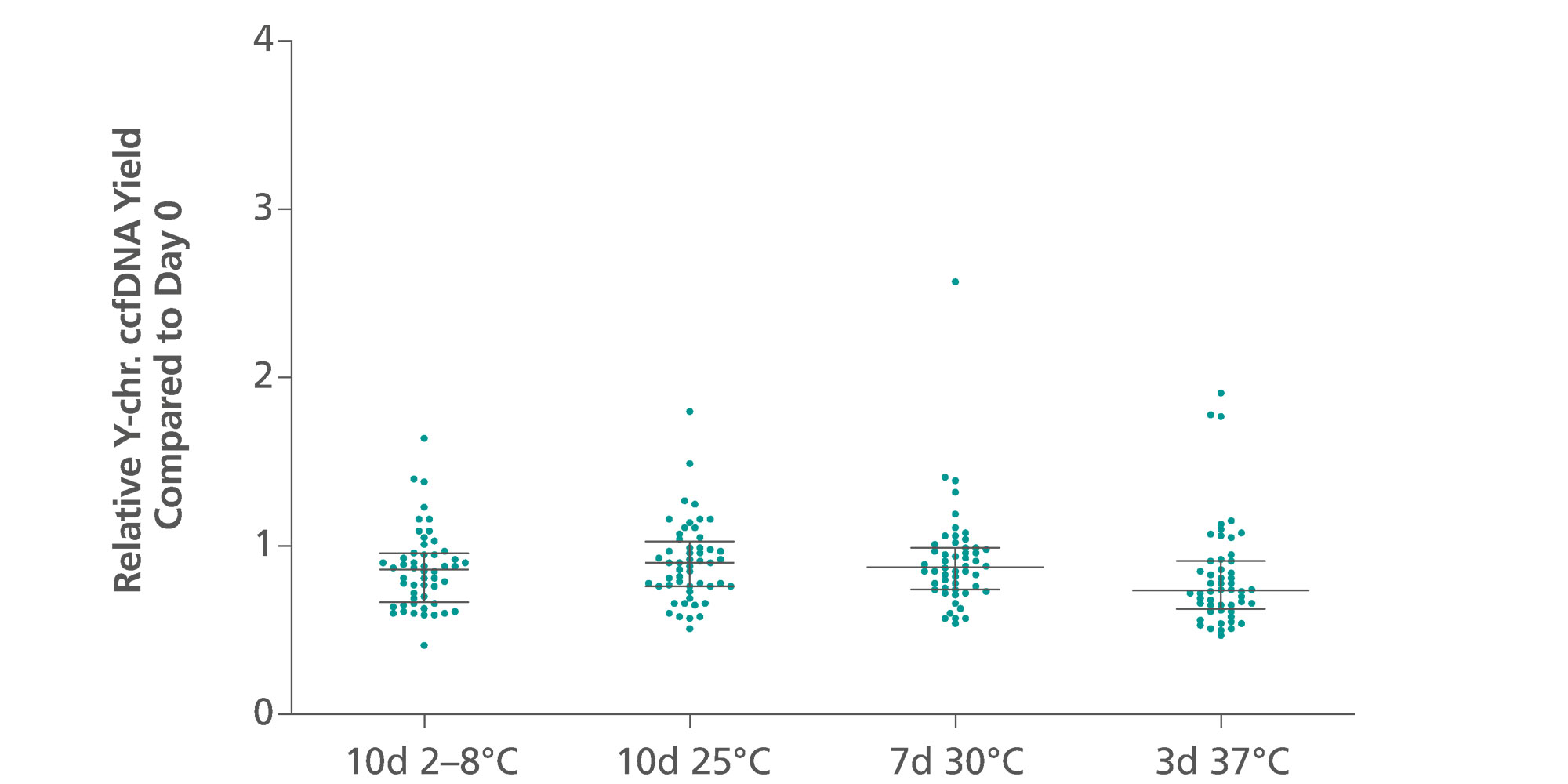
ccfDNA yields from plasma processed from blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) extracted with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) or the QIAamp DSP Circulating Nucleic Acid Kit are similar to plasma from EDTA tubes separated directly after blood draw (Figure 3). However, in contrast to unstabilized EDTA, blood drawn into PAXgene Blood ccfDNA Tubes have stable ccfDNA levels for up to 10 days at temperatures up to 25°C, 7 days at temperatures up to 30°C, or 3 days at temperatures up to 37°C (Note: Do not store blood-filled tubes below 2°C), because DNA release from cells lysing or apoptosis is inhibited (Figure 4) and ccfDNA is preserved (Figure 5).
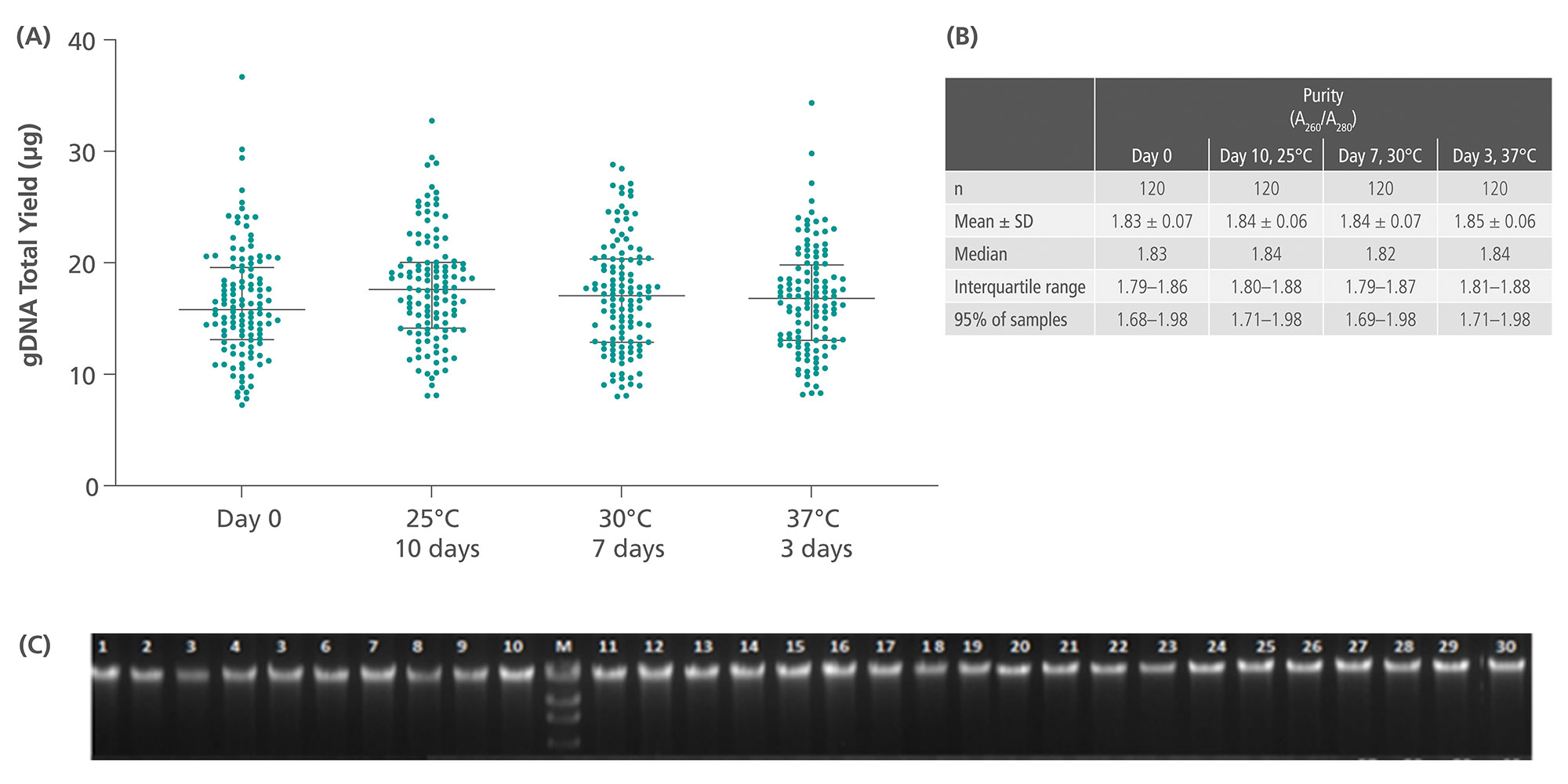
Genomic DNA in the nucleated cellular fraction is preserved and can be isolated with high purity, yield, and integrity (Figure 6). Thus, blood samples can be transported and stored within the specified temperature and duration to process plasma later (see technical note ´Sample Transportation Study (Summer Profile)´).
Plasma and nucleated cellular fraction generated from whole blood collected into PAXgene Blood ccfDNA Tubes are stable at room temperature for at least 3 days, at refrigerated temperature for at least 7 days and can be stored for long-term frozen at -20°C or -80°C (for latest update see technical note ´Plasma and Nucleated Cellular Fraction Stability Study´).
Pre-analytical steps including handling, storage, processing and documentation of verification and validation studies of PAXgene Blood ccfDNA Tube were conducted in accordance with ISO 20186-2:2019 and ISO 20186-3:2019: Molecular in vitro diagnostic examinations — Specifications for pre-examination processes for venous whole blood — Part 2: Isolated genomic DNA and — Part 3: Isolated circulating cell free DNA from plasma.
Stabilization characteristics have been shown to be robust against elevated level of endogenous, potentially interfering substances, underfilling and inappropriate mixing (see technical note ´Tube Robustness Study´).
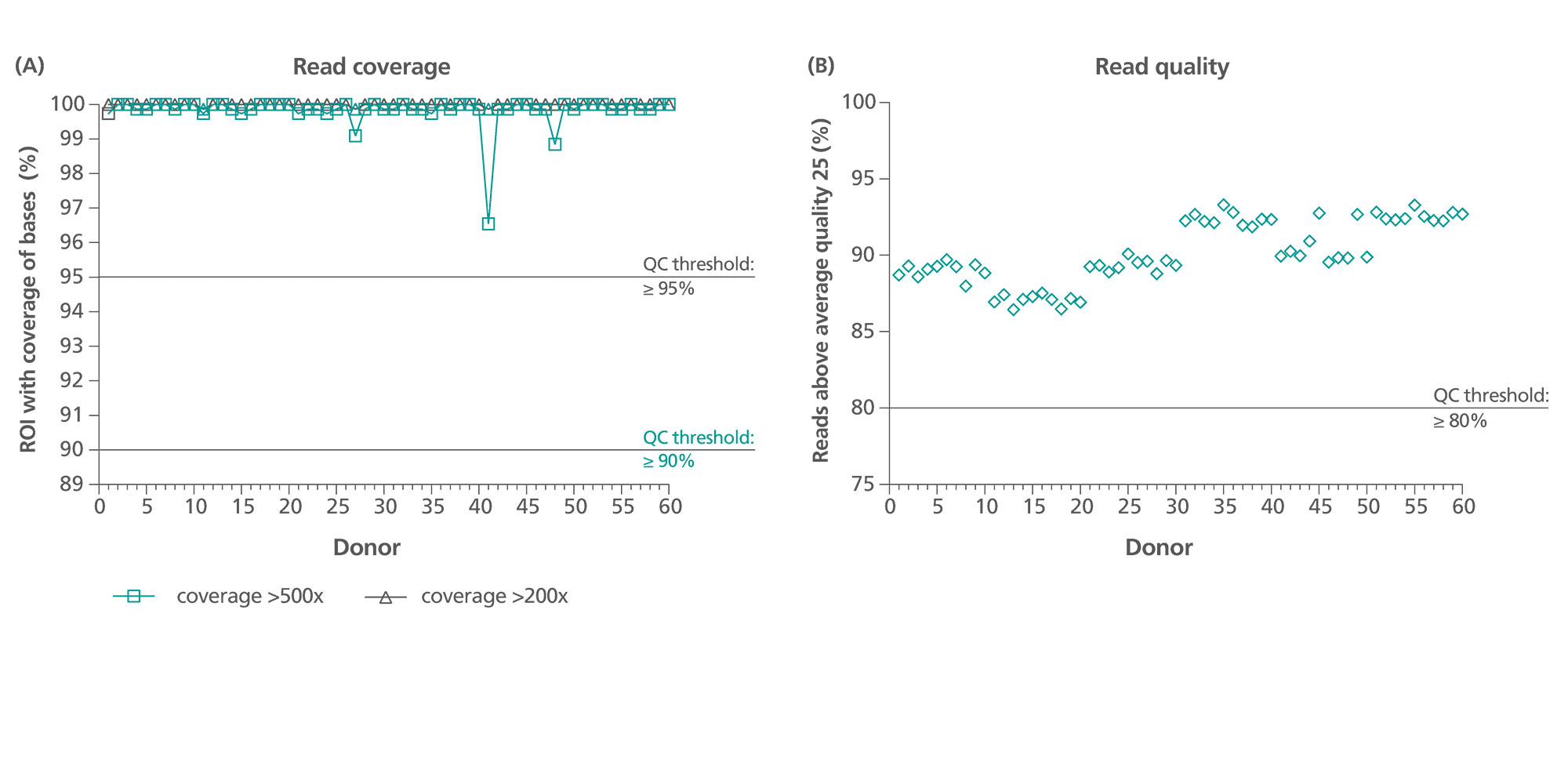
Both ccfDNA and gDNA yields are high-quality and are compatible for downstream analytical PCR assays, including user-validated methylation-based PCR, and next-generation sequencing (NGS) molecular test methods (Figure 7).
Principle
The PAXgene Blood ccfDNA Tube is intended for the collection, storage, and transport of human venous whole blood and stabilization of DNA for preparation of circulating, cell-free DNA (ccfDNA) from plasma and genomic DNA (gDNA) from the nucleated cellular fraction. The tube contains an additive that keeps blood from coagulating and inhibits apoptosis via a non-crosslinking formaldehyde-free stabilization solution. This helps prevent the release of intracellular DNA into the plasma and maintains constant ccfDNA levels during sample transport and storage before processing.
Procedure
(For details, see PAXgene Blood ccfDNA Tube (CE-IVD) Instructions for Use)
Blood is collected under a standard phlebotomy protocol into a closed, evacuated tube that contains a proprietary ccfDNA stabilization additive. Whole blood sample can be stored 10 days at temperatures up to 25°C, 7 days at temperatures up to 30°C, or 3 days at temperatures up to 37°C. Do not store blood-filled tubes below 2°C.
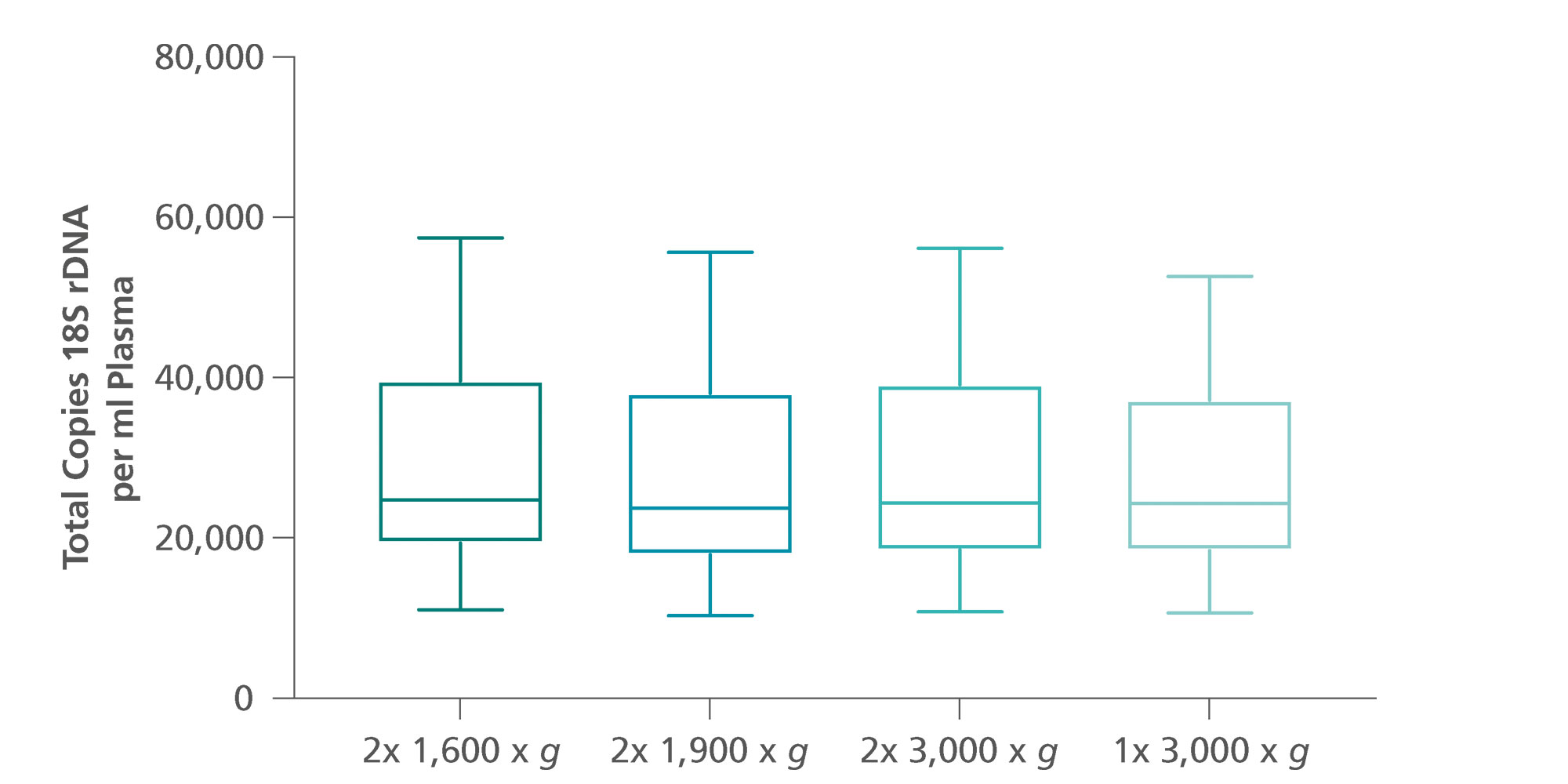
Centrifugation of blood collected into a PAXgene Blood ccfDNA Tube at room temperature (15–25°C) for 15 minutes at 1,600–3,000 × g leads to separation of plasma and nucleated cellular fraction. The cellular fraction includes a buffy coat, a leukocyte and platelet rich layer, and a red blood cell fraction. Plasma is removed after the first centrifugation into a secondary tube. For ccfDNA isolation, the plasma is centrifuged again for 10 minutes at 1,600–3,000 × g. As an alternative, after a first centrifugation for 15 minutes at 3,000 × g, the tube can be directly placed on the QIAsymphony SP instrument for automated ccfDNA extraction using the primary tube handling protocol of the QIAsymphony PAXgene Blood ccfDNA Kit (applies to 3,000× g only)(Figure 8).
After removal of the plasma, the remaining nucleated cellular fraction can be used to isolate gDNA.
Applications
ccfDNA purified from plasma generated from whole blood collected in PAXgene Blood ccfDNA Tubes (CE-IVD) and purified using the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) or QIAamp DSP Circulating NA Kit can be used in a wide range of downstream applications, such as:
- PCR, including digital, multiplex and quantitative real-time PCR
- Pharmacogenomic studies
- SNP genotyping
- Next-generation sequencing
- Methylation assays
PAXgene Blood ccfDNA Tube (CE-IVD) is for in vitro diagnostic use.