Sample Collection and Phlebotomy
1. Do I need to use a blood collection set with the PAXgene Blood ccfDNA Tube?
Yes. Further information on recommended phlebotomy technique with the PAXgene Blood ccfDNA Tube can be found in the Instructions for Use, accessible under the Resources tab on www.preanalytix.com.
2. Why is a blood collection set required to collect blood into the PAXgene Blood ccfDNA Tube?
When used in accordance with instructions, the blood collection set eliminates the possibility of patient contact with the reagent in the tube due to backflow of blood.
3. Can reagent from the PAXgene Blood ccfDNA Tube flow back into the patient's arm?
If the instructions for use are followed, there is no possibility of backflow during phlebotomy and thus, no possibility of reagent going into the patient's arm.
4. Can I use another manufacturer’s blood collection set with the PAXgene Blood ccfDNA Tube?
Yes, as long as the recommended phlebotomy instructions included in the PAXgene Blood ccfDNA Tube Instructions for Use are properly followed.
5. What is the blood draw volume of the PAXgene Blood ccfDNA Tube?
The blood draw volume is 10 ml and the stabilization additive volume is 1.5 ml. Thus, a filled tube holds a volume of 11.5 ml.
6. What is the additive in the PAXgene Blood ccfDNA Tube?
The PAXgene Blood ccfDNA Tube contains 1.5 ml liquid additive with a proprietary formulation that prevents blood coagulation and the release of genomic DNA from blood cells into plasma. Contents of the tube may cause irritation to the eyes, respiratory system and skin. Please refer to the PAXgene Blood ccfDNA Tube Instructions for Use, accessible under the Resources tab on www.preanalytix.com, for more details. Additional information can be found in the Safety Data Sheet, also accessible under the Resources tab.
7. What is the shelf life of unused PAXgene Blood ccfDNA Tubes?
The expiration date is printed on the tube label and on the shelf carton.
8. How should unused PAXgene Blood ccfDNA Tubes be stored?
Store the unused PAXgene Blood ccfDNA Tubes at 4–25°C and protect them from direct sunlight.
9. Are PAXgene Blood ccfDNA Tubes sterile?
Yes, the interior of the PAXgene Blood ccfDNA Tube is sterile. The sterilization method used is gamma irradiation.
10. Can I use underfilled PAXgene Blood ccfDNA Tubes?
A complete 10 ml blood draw ensures optimal results. Incomplete draw will change the blood to additive ratio and might have an impact on the downstream application.
11. The PAXgene Blood ccfDNA Tube additive has a slightly yellow appearance; does this affect the performance of the additive?
No, a slightly yellow appearance does not affect the performance of the additive. Do not use tubes after their expiration date.
12. The PAXgene Blood ccfDNA Tube has a slightly blue-gray tint; does this affect the performance of the tube?
No, a slightly blue-gray tint does not affect the performance of the tube. Do not use tubes after their expiration date.
Blood Stabilization and Storage
1.How does the additive in the PAXgene Blood ccfDNA Tube stabilize ccfDNA?
The stabilization additive aids in preventing blood coagulation and apoptosis of white blood cells.
2. Does the stabilization additive in the PAXgene Blood ccfDNA Tube contain formaldehyde or formaldehyde-releasing substances?
No. The stabilization additive in PAXgene Blood ccfDNA Tubes is completely free of formaldehyde or formaldehyde-releasing substances. It does not chemically modify or crosslink DNA from plasma or white blood cells.
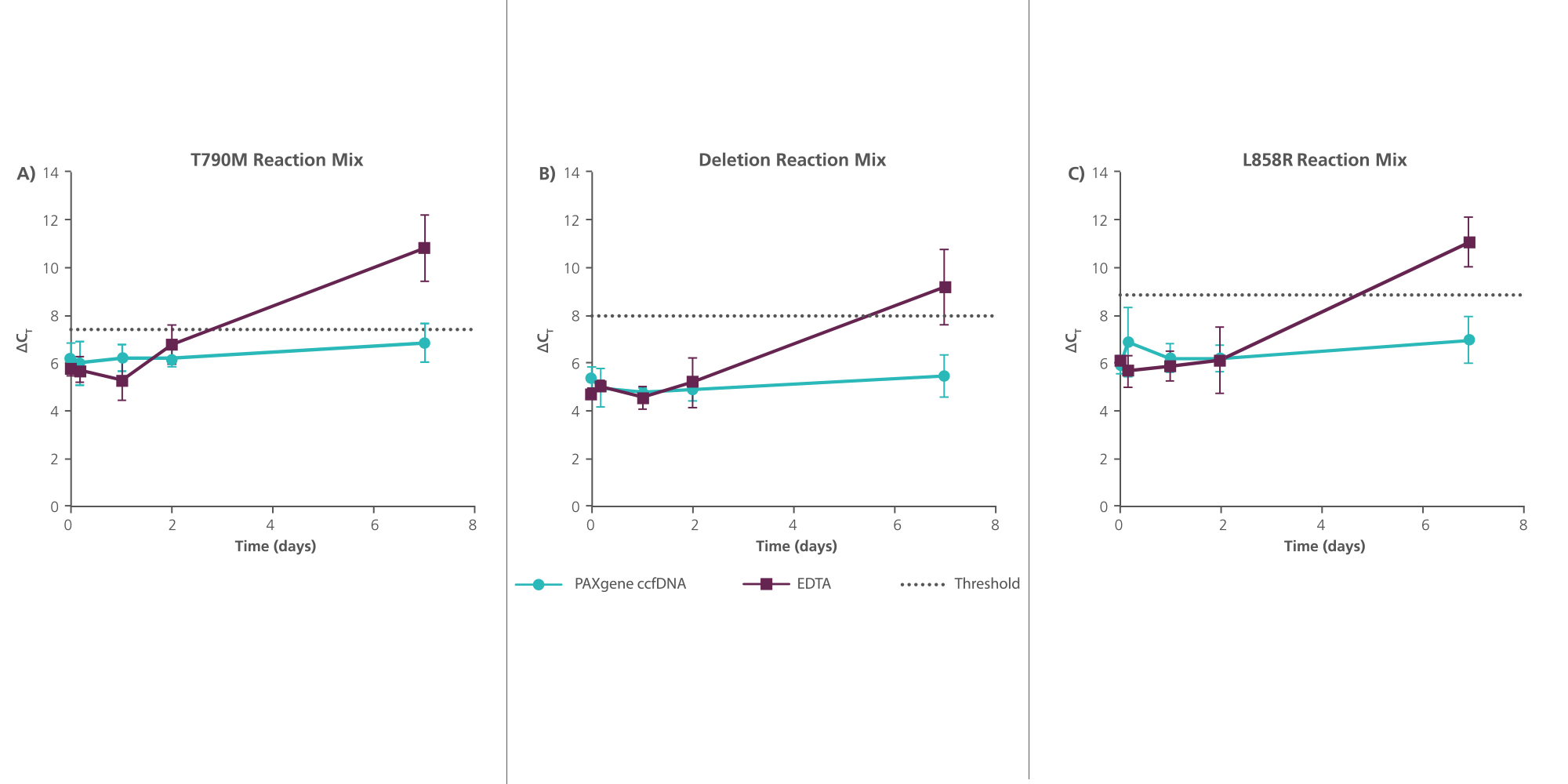
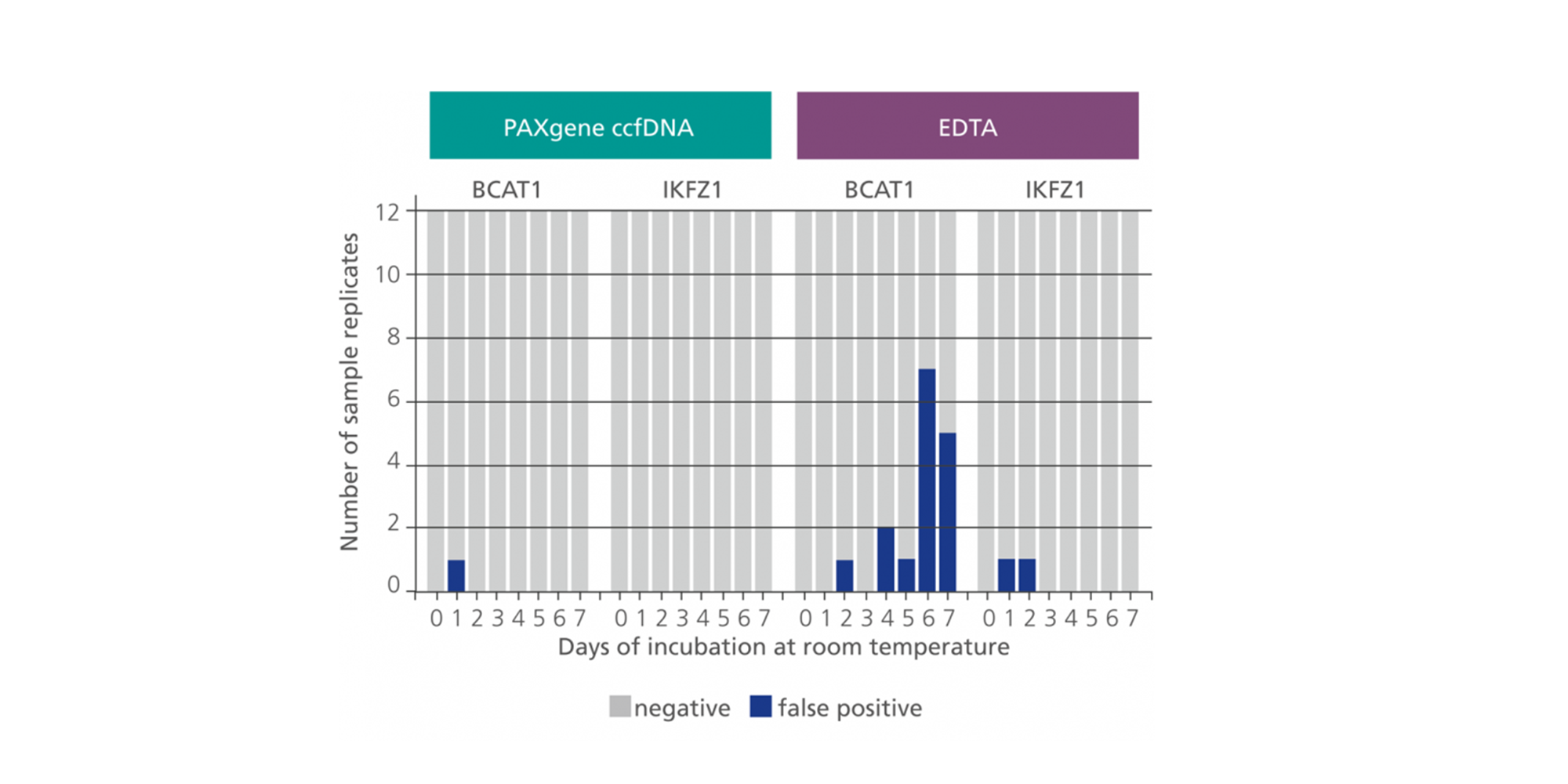
3. How long can whole blood stabilized in PAXgene Blood ccfDNA Tubes be stored at room temperature?
Whole blood drawn into PAXgene Blood ccfDNA Tubes can be stored at 2–25°C for up to 10 days.
4. Can whole blood in PAXgene Blood ccfDNA Tubes be stored at temperatures above 25°C?
Yes. Whole blood drawn into PAXgene Blood ccfDNA Tubes can be stored for up to 7 days at 2–30°C or up to 3 days at 2–37°C. Higher temperatures than 37°C or longer storage than indicated may lead to lysis of blood cells and release of genomic DNA into plasma.
5. Can whole blood in PAXgene Blood ccfDNA Tubes be stored at temperatures below 15°C? Yes. Whole blood drawn into PAXgene Blood ccfDNA Tubes can be stored below 15°C for up to 10 days. Storing blood in PAXgene Blood ccfDNA Tubes below room temperature does not extend stability. Do not store blood-filled tubes below 2°C.
6. Is it advantageous to store whole blood in PAXgene Blood ccfDNA Tubes at low temperatures (i.e., 2–8°C) compared to room temperature? PAXgene Blood ccfDNA Tubes can be stored at 2–8°C. However, storing blood in PAXgene Blood ccfDNA Tubes below room temperature does not extend stability.
Blood stored at refrigerated temperatures should be equilibrated to room temperature before processing.
7. Can whole blood be frozen in PAXgene Blood ccfDNA Tubes? No. Freezing blood drawn into PAXgene Blood ccfDNA Tubes results in lysis of red and white blood cells and release of genomic DNA.
8. Is genomic DNA stabilized in whole blood collected into PAXgene Blood ccfDNA Tubes? Yes. Genomic DNA is preserved and protected from apoptotic fragmentation.
Plasma processing
1.Is double centrifugation required for plasma processing?
An optional second centrifugation step helps remove traces of buffy coat that may have been accidently transferred when aspirating plasma after the first centrifugation step.
2. How long and at what temperature can plasma generated from PAXgene Blood ccfDNA Tubes be stored?
Plasma generated from whole blood collected into PAXgene Blood ccfDNA Tubes can be stored at room temperature (15–25°C) for up to 3 days or at 2–8°C for up to 7 days. We recommend freezing plasma at −20°C or −80°C for longer storage periods. Cell-free DNA (ccfDNA) is stable in frozen plasma for at least 12 months (long-term storage studies are ongoing).
3. How should I thaw frozen plasma samples?
Thaw plasma at room temperature (15–25°C). To minimize the formation of cryoprecipitates, do not thaw plasma at lower temperatures (e.g., 4°C). Alternatively, thaw plasma for 30 minutes at 30°C to completely avoid the formation of cryoprecipitates.
4. What should I do if cryoprecipitates form while thawing a frozen plasma sample? If cryoprecipitates form in the thawed plasma, vortex the tube for 30 seconds.
If plasma is processed with the PreAnalytiX QIAsymphony PAXgene Blood ccfDNA Kit, proceed according to the instructions provided with the kit.
If plasma is processed manually with the QIAGEN QIAamp Circulating Nucleic Acid Kit, cryoprecipitates will dissolve during lysis and incubation with Proteinase K.
Do not centrifuge the plasma to remove cryoprecipitates because they may contain ccfDNA.
5. How often can I freeze and thaw a plasma sample? Frozen plasma generated from a PAXgene Blood ccfDNA Tube is robust against at least three freeze/thaw cycles.
6. How should I adjust the braking step of the centrifuge for plasma processing? We recommend setting the braking to a medium level. No braking is possible but will unnecessarily prolong processing time. Setting braking level to a maximum is not recommended since it may lead to an angled border between plasma and the cellular fraction, which in turn may impede aspiration of the plasma.
7. How long and at which temperature can the nucleated cellular fraction from whole blood in PAXgene Blood ccfDNA Tubes be stored? Once plasma is aspirated the remaining nucleated cellular fraction in the PAXgene Blood ccfDNA Tubes can be stored at room temperature (15–25°C) for up to 3 days or at 2–8°C for up to 7 days. We recommend freezing nucleated cellular fraction at −20°C or −80°C for longer storage periods in a cryovial. Genomic DNA (gDNA) in frozen cellular fraction is stable for at least 12 months (long-term storage studies are ongoing).
8. How often can I freeze and thaw a cellular fraction sample? Frozen cellular fraction generated from a PAXgene Blood ccfDNA Tube can be frozen and thawed 3 times prior to DNA purification.
ccfDNA isolation and processing
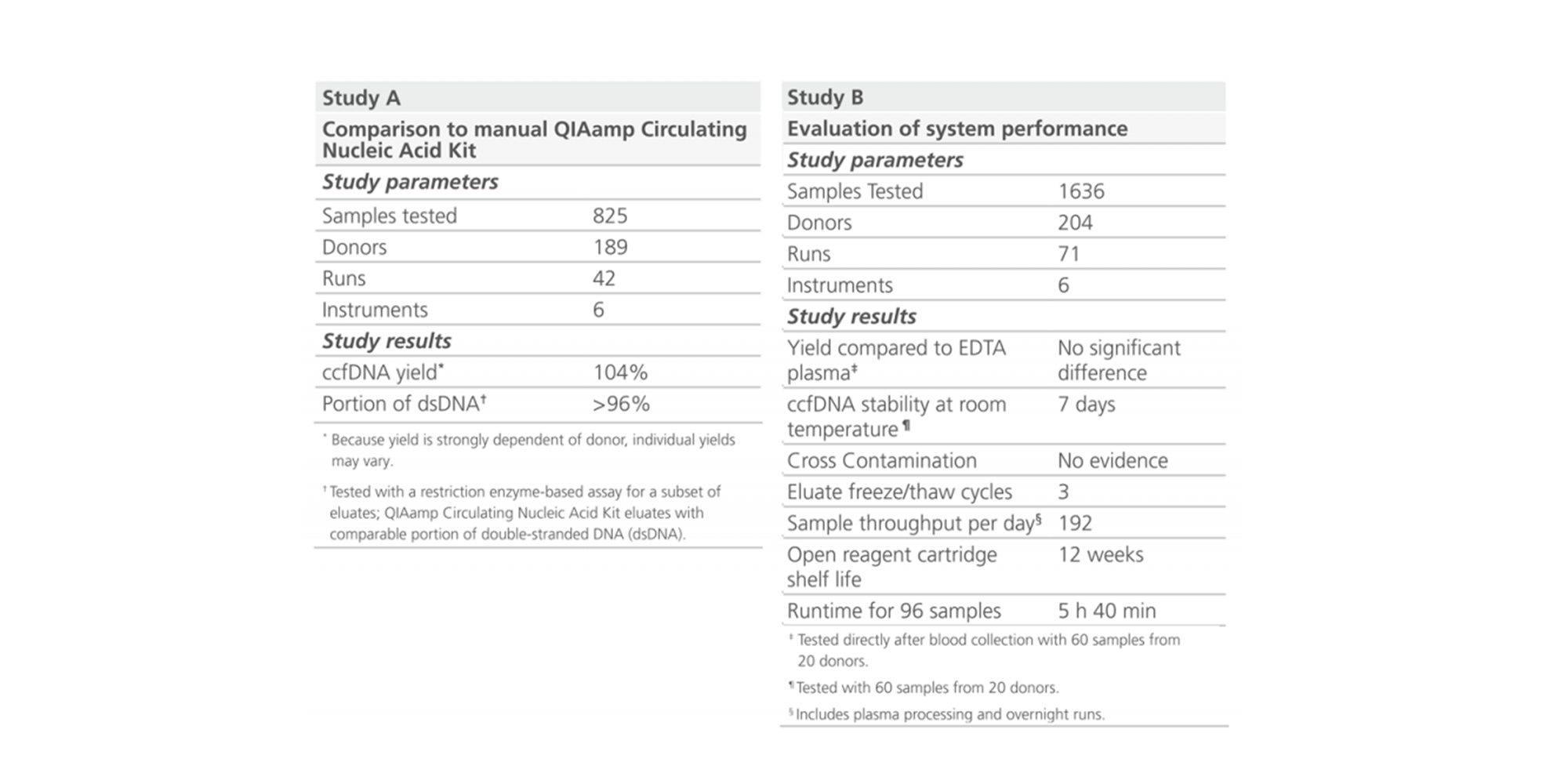
1. What is the expected ccfDNA yield from plasma generated from 10 ml blood?
The yield of ccfDNA depends on a number of preanalytical variables and is highly donor-dependent. Yields of ccfDNA are normally low in healthy donors, within the range of 5–100 ng/ml human plasma, but can drastically increase in the case of samples from cancer patients.
If similar input volumes of blood are used, ccfDNA yields from plasma processed from blood collected into PAXgene Blood ccfDNA Tubes will be similar to plasma from a spray-dried K2EDTA tube separated directly after blood draw.
However, in the PAXgene Blood ccfDNA Tube, ccfDNA yield remains stable for up to 10 days at temperatures up to 25°C, 7 days at temperatures up to 30°C, or 3 days at temperatures up to 37°C. In contrast, genomic DNA is released from lysed and apoptotic cells in unstabilized EDTA blood soon after blood draw.
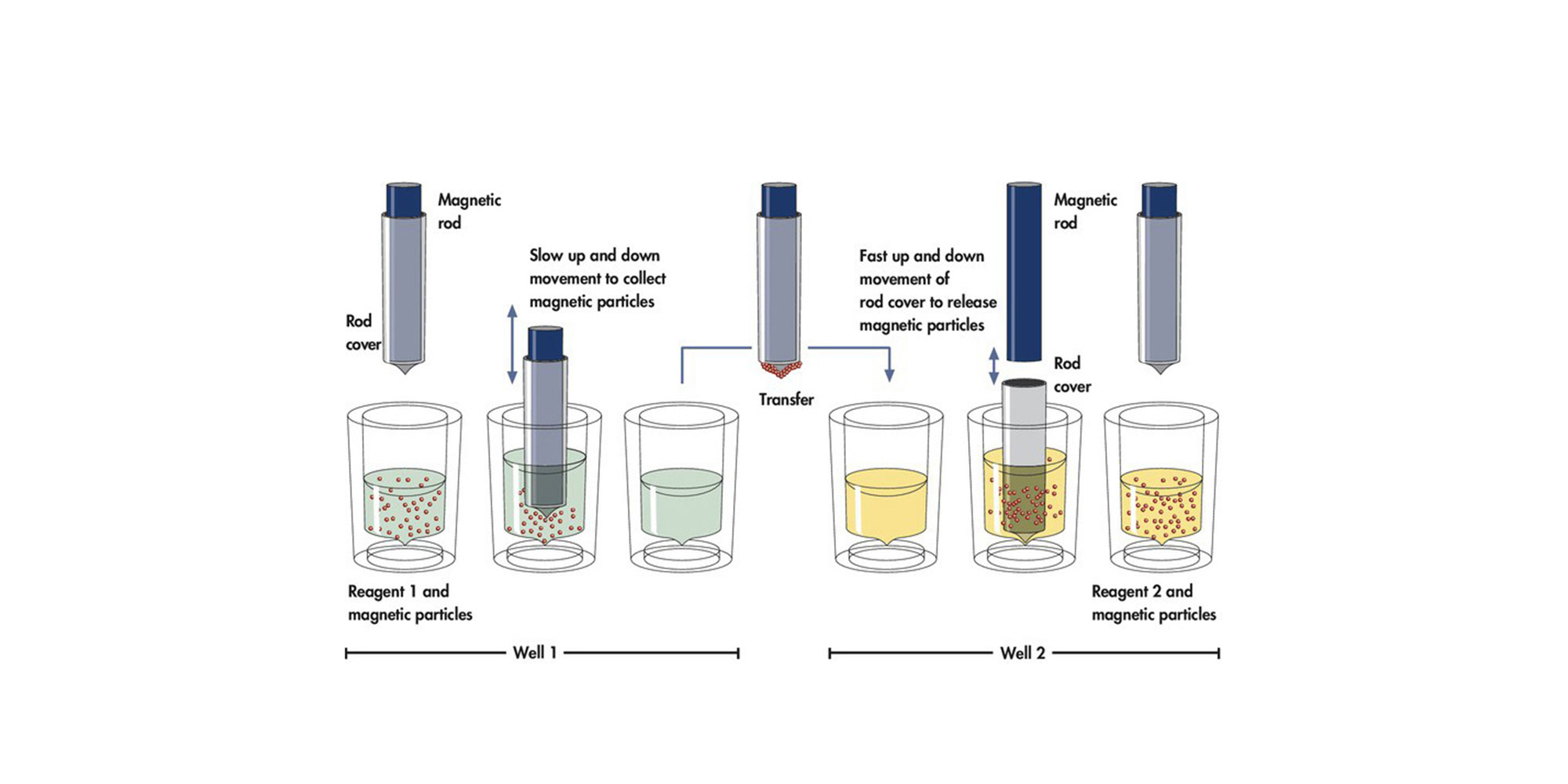
2.Which ccfDNA extraction kits are compatible with the PAXgene Blood ccfDNA Tube?
ccfDNA purification from the PAXgene Blood ccfDNA Tube is optimized when using the QIAsymphony PAXgene Blood ccfDNA Kit on the automated QIAGEN QIAsymphony SP instrument.
In addition, QIAGEN’s EZ1 ccfDNA kits can be used on the QIAGEN EZ1 Advanced XL instrument.
ccfDNA can also be purified manually with the QIAamp Circulating Nucleic Acid Kit or the QIAGEN QIAamp MinElute ccfDNA Mini and Midi Kits.
3. Are there any protocol modifications necessary when purifying ccfDNA manually with one of the QIAGEN QIAamp kits?
No. Plasma generated from a PAXgene Blood ccfDNA Tube can be used according to the instructions in the QIAamp kit handbooks. Since the stabilization additive is free from formaldehyde or formaldehyde releasing substances, no prolonged proteinase K digestion or any other protocol modification is necessary.
4. Which plasma volumes can be processed on the QIAsymphony SP instrument using the QIAsymphony PAXgene Blood ccfDNA Kit?
In the standard protocols, sample input volumes of 2.4 ml or 4.8 ml plasma can be selected. Because of void volumes of 0.4 ml and 0.5 ml, a minimum of 2.8 ml and 5.3 ml sample input respectively must be placed onto the instrument in a secondary tube.
In the primary tube handling protocols, input volumes of 2.4 ml or 4.0 ml plasma can be selected. Plasma volumes qualified for primary tube handling are measured with the PAXgene Blood ccfDNA Purification Protocol Selection Tool provided in the kit.
5. Is it possible to process less than 2.4 or 4.8 ml of plasma with the standard protocols on the QIAsymphony SP instrument using the QIAsymphony PAXgene Blood ccfDNA Kit?
Yes. Plasma volumes lower than the 2.8 ml or 5.3 ml needed for standard protocols (2.4 ml or 4.8 ml plus void volume) can be used. The “Less Sample mode”, an integrated part of the protocol function, allows the transfer of lower plasma volumes by the instrument. In the result file the discrepancy between the regular and the transferred plasma volume is documented. The minimum plasma input volumes to enable “Less Sample mode” are 1.6 ml or 4.1 ml.
6.How many extractions can I perform with a QIAsymphony PAXgene Blood ccfDNA Kit?
The number of extractions that can be performed with a kit is independent of sample volume and ccfDNA extraction protocol. A kit contains sufficient reagents for 192 extractions.
7. What is the sample throughput per working day (8h) and QIAsymphony instrument?
Sample throughput is 192 samples (2 x 96 samples) from plasma generation to the start of the second run (overnight run) with 96 samples.
8. How long after first use can a reagent cartridge from a QIAsymphony PAXgene Blood ccfDNA Kit be stored for further use?
When closed properly with the provided strips and stored at room temperature (15–25°C), a reagent cartridge can be used up to 4 weeks after the initial use.
9.What is the shelf life of the QIAsymphony PAXgene Blood ccfDNA Kit?
The expiration date is printed on the kit label and encoded in the bar code of the reagent cartridges.
10. For what type of sample material is the QIAsymphony PAXgene Blood ccfDNA Kit intended?
The QIAsymphony PAXgene Blood ccfDNA Kit is designed to be used with plasma generated from human venous whole blood collected into PAXgene Blood ccfDNA Tubes.
11.Which tubes are recommended for the QIAsymphony SP instrument sample rack? The recommended tube is the 14 ml Falcon® polystyrene round bottom tube, 17 x 100 mm, supplied by Corning (formerly supplied by BD).
For the primary tube handling workflow, the PAXgene Blood ccfDNA Tube, 16 x 100 mm, can be directly loaded into the sample rack after initial centrifugation. See labware list for more detailed information, available under product page on www.qiagen.com.
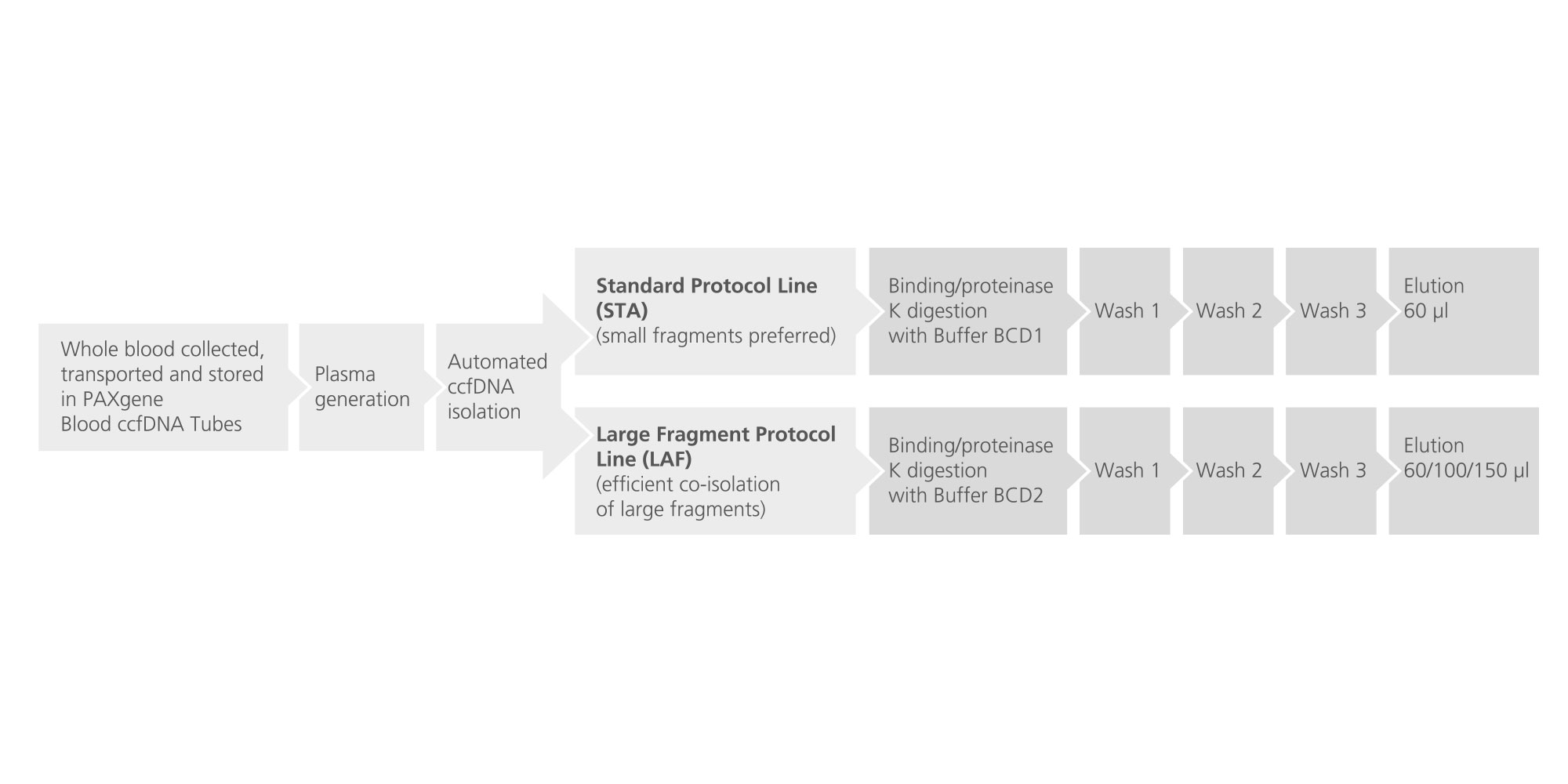
12. Which elution volumes for ccfDNA extraction are available for the QIAsymphony PAXgene Blood ccfDNA Kit? The elution volume of the standard protocols (STA) is fixed at 60 µl. The large fragment protocols (LAF) enable elution in 3 different volumes: 60, 100 and 150 µl.
13. How should eluates produced with the QIAsymphony PAXgene Blood ccfDNA Kit be stored? Eluates should be stored at −20°C or −80°C (long-term storage studies are ongoing).
14. Which protocol line should I use to process plasma samples? The QIAsymphony PAXgene Blood ccfDNA Kit can be used with 2 protocols. The standard protocols (STA) predominantly isolate small ccfDNA fragments. The large fragment protocols (LAF) efficiently co-purify large and small ccfDNA fragments. Both lines include protocols for 2.4 ml and for 4.8 ml plasma input volumes.
15. Does the QIAsymphony PAXgene Blood ccfDNA Kit also allow eluting ccfDNA in less than 60 µl? Yes, a customized protocol for elution in 50 µl is available upon request.
Downstream applications
1. Which downstream applications have been tested with ccfDNA purified from whole blood collected into PAXgene Blood ccfDNA Tubes?
To date, ccfDNA from blood samples collected and processed using the PAXgene Blood ccfDNA workflow have been successfully tested in PCR, including multiplex qPCR, dPCR and NGS applications.