

Our latest publication describes a blueprint test methodology for researchers to determine the potential impact of different ccfDNA profile stabilizing technologies on the sensitivity of their liquid biopsy assay.
This publication may be of interest to you if you are trying to detect ccfDNA targets at lower levels and maximize the sensitivity of your diagnostic assay, as we understand assay sensitivity is a key success factor.
This paper highlights the importance of choosing the ccfDNA stabilization chemistry during the early stages of assay development in order to maximize assay sensitivity.
Link to the paper:
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0253401
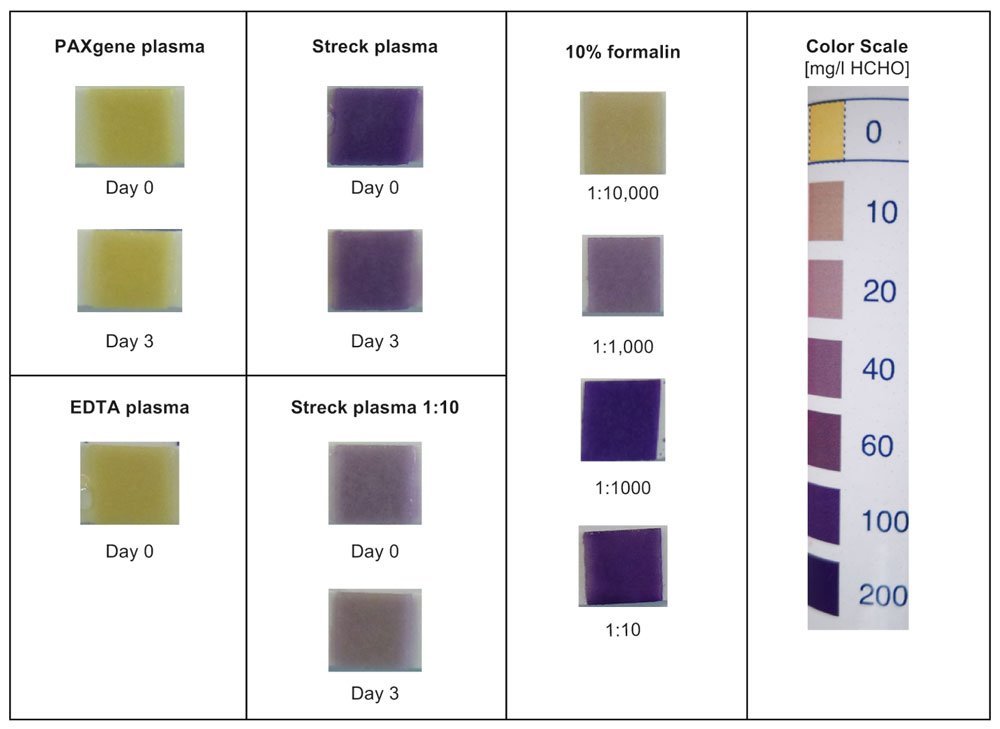
The results of the paper demonstrate that the choice of the blood collection tube (BCT) can have a direct impact on the sensitivity of an assay. The Streck Cell-Free DNA (cfDNA) BCT contains a formaldehyde-releasing additive whereas the PAXgene Blood ccfDNA Tube stabilizes blood with a non-crosslinking, formaldehyde-free chemistry. Further, the data shows that PAXgene Blood ccfDNA Tubes have a higher assay sensitivity. Additives in blood collection tubes may have an influence on the limit of detection (LOD) of your assay and investing in optimizing the start of your workflow may pay off in detecting more ccfDNA in downstream analysis.
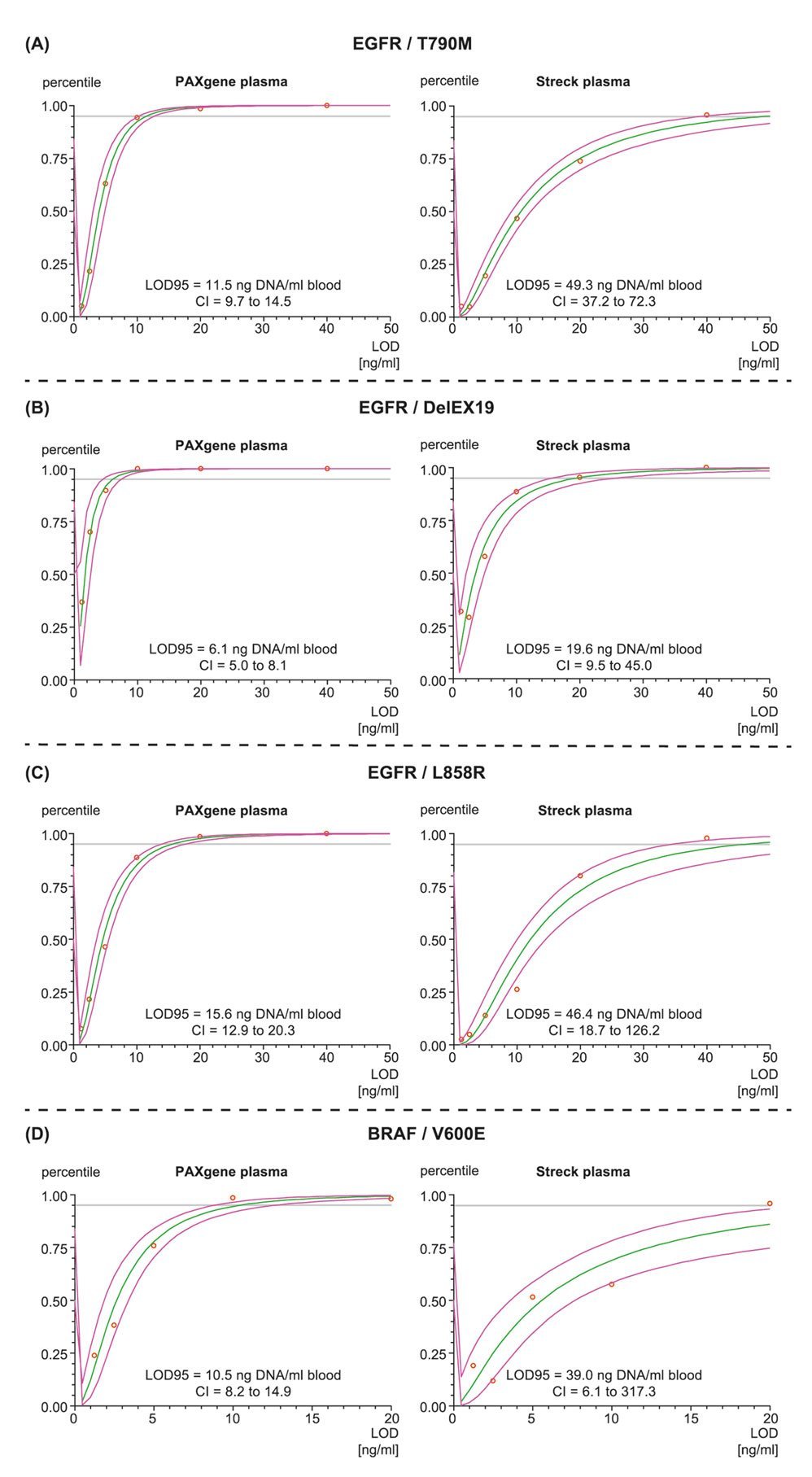
The paper describes in detail a workflow for detecting Epidermal Growth Factor Receptor, EGFR and BRAF mutations using either PAXgene Blood ccfDNA Tubes or Streck cfDNA BCTs. For both EGFR and BRAF assays, mutation hit rates were higher in samples collected and stabilized in PAXgene Blood ccfDNA Tubes compared to Streck samples. The workflow using PAXgene Blood ccfDNA Tubes resulted in a lower limit of detection (LOD) better assay sensitivity than the compared workflow and thus avoids missing critical mutant gene copies.

The publication provides a liquid biopsy study design which was carried out according to the ISO Standard 20186-3:2019 (Molecular in vitro diagnostic examinations–Specifications for pre-examination processes for venous whole blood–Part 3: Isolated circulating cell free DNA from plasma) and can be seen as a methodology for diagnostic laboratories to develop a standardized and optimized assay workflow. This study proposal can be viewed as a blueprint for verifying and validating a liquid biopsy test from blood collection to the analytical result, using established standards and guidelines to help reduce errors and improve accuracy and reliability of data.